
Special Article - Retinal Diseases
J Ophthalmol & Vis Sci. 2016; 1(1): 1003.
Prevention of Glaucoma-Induced Retinal Ganglion Cell Loss Using Alpha7 nAChR Agonists
Birkholz PJ¹, Gossman CA¹, Webster MK¹, Linn DM² and Linn CL¹*
¹Department of Biological Sciences, Western Michigan University, USA
²Department of Biomedical Sciences, Grand Valley State University, USA
*Corresponding author: Cindy Linn, Department of Biological Sciences, Western Michigan University, USA
Received: February 20, 2016; Accepted: March 28, 2016; Published: March 30, 2016
Abstract
In this study, the neuroprotective effect of various nicotinic alpha7 acetylcholine receptor agonists in an in-vivo model of glaucoma using adult Long Evans rats was analyzed. Glaucoma-like conditions were induced in the eyes of Long Evans rats after injection of hypertonic saline into episcleral veins to create scar tissue and increase the animal’s intraocular pressure. This procedure produced significant loss of retinal ganglion cells within one month and was associated with an increase of intraocular pressure. Using this model system, various alpha7 nicotinic acetylcholine receptor (a7 nAChR) agonists were applied at different doses as eye drops to the right eye of adult Long Evans rats while the left eye was left as an internal control. The a7 nAChR agonists used in this study prevented loss of RGCs in a dose dependent manner after the procedure to induce glaucoma-like conditions. PHA-543613 and PNU- 282987 provided the largest degree of RGC survival after inducing glaucomalike conditions, followed by nicotine, SEN 12333, tropisetron, 3-Bromocytisine and DMAB. To provide evidence that neuroprotection of RGCs was mediated through activation of a7 nAChR, in some studies different concentrations of the a7 nAChR antagonist, MLA, was intravitreally injected into experimentally treated eyes before initiation of eye drops and the procedure to induce glaucoma-like conditions. In the presence of MLA, RGC neuroprotection was blocked. Results from these studies suggest that selective a7 nAChR agonists may be used in future therapeutic treatments for glaucoma or other CNS diseases associated with a7 nAChRs.
Keywords: Alpha7 nicotinic acetylcholine receptors; Glaucoma; Retina; Retinal ganglion cells; Acetylcholine; Nicotinic agonists
Abbreviations
ACh: Acetylcholine; a7 nAChRs: Alpha7 Nicotinic Acetylcholine Receptors; CO2: Carbon Dioxide; DMAB: 4-[(5,6-Dihydro[2,3’- bipyridin]-3(4H)-ylidene)methyl]-N,N-dimethylbenzenamine dihydrochloride; DMSO: Dimethyl Sulfoxide; hERG: human Ether-a-go-go Related Gene; IACUC: Institutional Animal Care and Use Committee; IOP: Intraocular Pressure; KAX: Ketamine, Acepromazine and Xylaxine cocktail; LC MS/MS: Liquid Chromatography, Mass Spectroscopy, Mass Spectroscopy; ONH: Optic Nerve Head; PBS: Phosphate Buffer; RGCs: Retinal Ganglion Cells; NaCl: Sodium Chloride
Introduction
Glaucoma is one of the leading causes of blindness worldwide [1- 3]. It is a neurodegenerative disorder characterized by a progressive optic neuropathy, cupping of the optic disk, the death of RGCs and degeneration of axons in the optic nerve [4,5]. The primary risk factor associated with glaucoma is an increase in intraocular pressure, which has been linked to apoptosis of Retinal Ganglion Cells (RGCs) [6-8]. Recently, a great deal of research has explored the agents and mechanisms that provide neuroprotection against neurodegenerative conditions. Activation of alpha7 nicotinic acetylcholine receptors (a7 nAChRs) in the brain have been linked to neuroprotection against several neurodegenerative diseases [9,10]. There is strong evidence that a7 nAChRs are neuroprotective, reducing β-amyloid induced toxicity in Alzheimer’s disease [11,12] and that the a7 nAChRs plays a role in the pathophysiology of schizophrenia [13,14]. In the retina, RGCs contain a7 nAChRs [15-17] and receive cholinergic input from a well-described population of starburst amacrine cells [18,19]. They are the only source of ACh in the vertebrate retina. Previous studies have demonstrated that intravitreal injections or eye drop application of the a7 nAChR agonist, PNU-282987, in an in vivo rat model, prevented the loss of RGCs typically associated with a procedure to induce glaucoma-like conditions [20,21].
Binding studies in rat chimera cells using PNU-282987 have demonstrated that PNU-282987 is a potent specific agonist for a7 nAChRs [22]. These studies demonstrated that Methyllycaconitine (MLA), a specific a7 nAChR antagonist, preferentially bound to a7 nAChRs when both PNU-282987 and MLA were present. In electrophysiology studies using rat hippocampal neurons, PNU- 282987 evoked a rapidly desensitizing inward whole-cell current associated with the opening of the a7 nAChR channel. This current was eliminated if MLA was introduced before PNU-282987 [22].
Although PNU-282987 has been used in animal schizophrenia models, it has not been found to be suitable for systemic use in humans because of excessive inhibition of a hERG anti target in the heart [23]. As a result, other a7 nAChR agonists were investigated to determine their neuroprotective effect following topical delivery in a rat glaucoma model. Comparisons between the neuroprotective effect of PNU-282987 and the other a7 nAChR agents are discussed. The results from these studies support the hypothesis that a7 nAChR agonists may have a role in the future as a therapeutic intervention for glaucoma.
Materials and Methods
Animals
Both male and female adult Long Evans rats were used in this study. Rats were used at 3 months of age. Out bred Long Evans rats were chosen because of their docile nature, pigmented eyes, the lack of genetic defects that are associated with other inbred rat species and for consistency with studies already completed [20,21]. All animals were cared for in accordance with the approved guidelines of the Institutional Animal Care and Use Committee (IACUC) at Western Michigan University.
Anesthesia
To anesthetize Long Evans rats, animals were injected intraperitoneally with 1.0 ml/kg KAX standard rat cocktail; consisting of a solution of 5 ml of ketamine (100 mg/ml), 2.5 ml of xylazine (20 mg/ml), and 1 ml of acepromazine (10 mg/ml) in 3 ml sterile water. KAX was injected prior to hypertonic saline injections to induce glaucoma and prior to intravitreal injections (if utilized in the experiment). To ensure complete anesthesia, a lack of tail and toe pinch reflex were needed before proceeding with procedures. After the procedures were completed, rats were placed on a warm circulating water pad and watched until they were fully awake and functioning normally before being returned to the animal colony.
Procedure to induce glaucoma
To induce glaucoma, rats were anesthetized with an intraperitoneal injection of KAX and two drops of pilocarpine were added to the right eye of each rat to prevent the blinking reflex. After applying betadine solution around the eye, a hemostat was used to pinch the skin surrounding the eye to bulge the eye from the eye socket and reveal the episcleral vein for hypertonic saline injection. The right eye in each animal was used for procedural manipulation while the left eye served as an untreated internal control for each experiment unless noted otherwise. Under a dissection microscope, the episcleral vein of the right eye was injected with 50 μL of 2M NaCl using a micro needle assembly [20]. Injection of 2M NaCl caused blanching of the vein, which ensured a successful injection. Observation of a blanched vein correlated to a significant loss of RGCs one month following the procedure [20]. Following the injection, a small amount of antibiotic ointment was applied over the injection site and where the hemostat pinched the skin around the eye. This procedure was modified from the procedure originally developed by Morrison et al. [24]. In sham procedure studies, injections were made with PBS instead of ACh agonists.
IOP measurements using Tonopen
IOP measurements were obtained before hypertonic injections to obtain a baseline and after hypertonic injections into the episcleral veins to measure any increase of IOP. IOP measurements were obtained from awake behaving rats using the Tono-Pen XL tonometer (Mentor, Norwell MA) according to instructions outlined by Morrison et al. [24]. Rat eyes were anesthetized with 1 drop of 0.5% proparacaine hydrochloride before using the Tono-Pen. Rats were loosely held in the experimenter’s hand during this procedure. IOP measurements were obtained each day for 1 week before hypertonic injections and 5 times each week after hypertonic injections until the rats were sacrificed [20].
Eye treatments
Previous in vivo studies on adult Long Evans rats have shown that the a7 nAChR specific agonist, PNU-282987, prevented the loss of RGCs typically associated with inducing glaucoma-like conditions when applied as eye drops [21]. Other studies have also demonstrated that pharmacological agents can reach the retina if the agents are dissolved in appropriate vehicles [25-27]. In this study, the neuroprotective effect of the following commercially available a7 nAChR agonists were examined, including PHA-543613, PNU- 282987, nicotine, SEN 12333, tropisetron, 3-bromocytisine and DMAB. Three different concentrations of each agent were used for dose-response studies. The concentrations of agents used were based on previously obtained preliminary results. ACh agonists were dissolved in Dimethyl Sulfoxide (DMSO) to make a stock solution and then diluted in PBS and sterilized using syringe filters. 30 μl eye drops were applied for three days to the bulbar conjunctiva of the eye before the injection of hypertonic solution to induce glaucomalike conditions and for one month following the procedure [20,21]. Eye drops were applied twice a day based on previous LC MS/MS studies that demonstrated evidence of PNU-282987 in the retina up to 12 hours after treatment [20]. Between 4 and 20 animals were used for each experiment. The left eye in each animal was untreated and served as an internal control to compare against the experimentally treated right eye. After one month, animals were sacrificed by C02 asphyxiation and retinas were removed for RGC labeling. Vehicle control experiments were performed concurrently using only PBS eye drops (N=4), or eye drops containing up to 1% DMSO (N=4).
To support the hypothesis that the ACh agonists act through a7 nAChRs, experiments were performed using a specific a7 nAChR antagonist, MLA. In these experiments, 5 μL of various concentrations of the a7 nAChR specific antagonist, MLA (0, 1, 10, 100nM) (N=4 for each concentration),was injected directly into the vitreal chamber of the right eye using a Hamilton syringe 1 hour before initiation of eye drop treatments and before the procedure to induce glaucoma-like conditions.
Tissue preparation and analysis
One month following the hypertonic saline injection, rats were euthanized using CO2 asphyxiation. Both eyes were subsequently removed and processed [20]. After removal of the cornea, lens and vitreous humor, the whole retina was peeled away from the back of the eye cup. Care was taken to remove the retina in one piece to maintain geographical orientation and landmarks. Once the whole retinas were removed, four short evenly spaced slits were made around the retina’s periphery that allowed the retinas to be flattened. The retina was then pinned out flat onto sylgard plates using cactus needles and fixed in 4% paraformaldehyde for 24 hours at 4oC. The next day, retinas were rinsed, blocked and permeabilized with 2% bovine serum and 1% triton X-100 (Sigma) for two hours at room temperature. Retinas were then immunostained with antibodies against Thy 1.1 or double-labeled with antibodies against Thy 1.1 and Brn3a. Anti Thy 1.1 (mouse anti-rat, BD Biosciences) is a monoclonal antibody against glycoproteins found exclusively on the plasma membrane of RGCs in the retina [28]. Anti-Brn3a is an antibody that labels the transcription factor found in RGCs [29]. For single labeling of RGCs, the primary antibody, mouse anti-Thy1.1 (1:300, BD Pharmingen) was added to the flat-mounted retina and incubated overnight on a rocker, in a humidified chamber at room temperature. 24 hours later, retinas were rinsed twice in 0.1% Triton X-100 and three times in PBS. The secondary antibody, Alexa Fluor 594 goat anti-mouse, was then applied in PBS (1:300, Life technologies) and the retinas were incubated for 24 hours on a rocker, in a darkened humidified chamber at room temperature. After incubation in secondary antibody, the cactus needles were removed and the retinas were transferred to glass slides and mounted using 50% glycerol and 50% PBS. When double-labeling RGCs, anti-Thy 1.1 and rabbit anti-Brn3a (1:300) were used in PBS containing 2% bovine serum. The secondary antibodies used to visualize double labeled RGCs were Alexa Fluor 594 goat anti-mouse and Alexa Fluor 488 donkey anti-rabbit (1:300, Life technologies) [20].
Once stained, the number of single or double-labeled RGCs throughout the RGC layer at specific regions of the retina was counted from images obtained 4 mm from the Optic Nerve Head (ONH) using the Z-stack capabilities of a Nikon confocal microscope. In each retina, 200 μm2 images were obtained from the dorsal retina containing the highest density of RGCs in the visual streak, the ventral, nasal and temporal regions of the retina [20]. All images were obtained 4 mm from the ONH as a previous study demonstrated that the greatest loss of RGCs occurred in the peripheral retina under glaucoma conditions [20]. The number of RGCs counted from each experimentally treated retina were averaged and compared directly to the number of RGCs obtained from internal control retinas and normalized by calculating the percent change from each internal control. Four to twenty different rats were used for each experimental condition and in control studies to determine if the delivery method or vehicle had any effect on RGC counts. In other control studies, experiments were conducted to display specificity of the antibodies used. In four negative control experiments, retinas were processed with the primary antibodies omitted, while another four experiments substituted non-immune mouse immunoglobulin (dilution: 0.1 - 1.0 μg/ml) for the antibodies. In other experiments, pre absorption controls were performed where the primary antibody and antigens were added together before applying to tissue (N=4). No significant epifluorescence was observed under any of these conditions.
Statistical analysis
For normalized data, statistical analysis was performed using Kruskall-Wallis non parametric analysis of variance with post hoc comparisons (Dunn’s test). P-values of < 0.05 represented significance. Graphs were plotted with Prism GraphPad version 4.0 software (GraphPad Software, Inc., San Diego, CA). All data values are reported as the mean ± Standard Error (S.E.). Errors bars are presented one-sided to prevent error bar clutter in dose-response studies and for consistency.
Results
Glaucoma effects on RGCs
The primary risk factor associated with glaucoma is an increase in Intraocular Pressure (IOP) [30-32]. Earlier studies from this lab using the Tono-Pen XL tonometer (Mentor, Norwell MA) have demonstrated that injection of hypertonic saline into Long Evans rat’s episcleral veins significantly increased intraocular pressure from an average of 13 mm Hg before the procedure to induce glaucoma to an average of 21 mm Hg within one month afterward [20]. Corresponding with this increase of intraocular pressure is a significant loss of RGCs. (Figure 1) illustrates the typical loss of RGCs that result from 50 μl injection of 2M NaCl into the episcleral vein of adult anesthetized Long Evans rats. (Figure 1A) is an image obtained from the left control untreated eye of a flat-mounted retina doublelabeled with antibodies against the glycoprotein, Thy 1.1 and the RGC nuclear marker, Brn3a. Images were obtained from the nerve fiber layer and the RGC layer using a Nikon confocal microscope. The antibody against Thy 1.1clearly labeled the plasma membrane of RGC bodies (arrows) when secondarily labeled with Alexa Fluor 594. Anti-Brn3a also labeled RGCs and was found to be co-localized in cell bodies of the RGC layer that labeled with Thy 1.1. The anti-Brn3a antibody was secondarily labeled with Alexa Fluor 488. Co-labeled RGCs occurred 95.5% (+/-3.5; N=5) of the time.
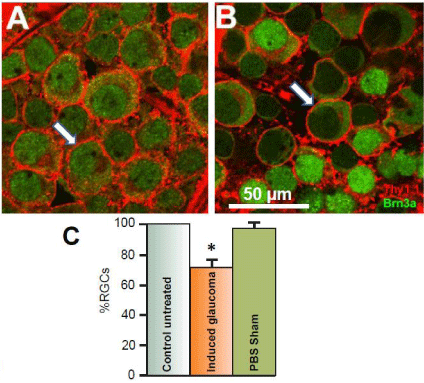
Figure 1: Effects of the glaucoma-inducing procedure on RGC survival.
Figure 1A represents a confocal image obtained 4 mm from the optic nerve
head in a control untreated retina after double-labeling RGCs with antibodies
against Thy 1.1 (red) and Brn3a (green). Thy 1.1 stains glycoproteins found
in the plasma membrane of each RGC (red), while Brn3a is a nuclear
marker for RGCs (green). Figure 1B represents an image obtained from the
experimental eye of the same animal after hypertonic saline was injected into
the episcleral vein to induce glaucoma-like conditions. The image shown was
obtained one month after the procedure and was obtained from the same
retinal location as the image shown from the internal control eye. Arrows
indicate RGCs. The bar graphs in figure 1C represent the average percent
survival of RGCs compared to internal controls under different conditions.
To generate the sham results, PBS was injected into the episcleral veins
instead of hypertonic saline. The star represents significant difference from
the control untreated condition. Error bars represent S.E.
Figure 1B illustrates a confocal retinal image obtained from the right eye of the same animal; one month after hypertonic saline was injected into the animal’s episcleral vein. Both images in (Figures 1A & 1B) were obtained from the same geographical location in the retina, 4 mm from the ONH. As seen in (Figure 1B), there was significant loss of RGCs after inducing glaucoma-like conditions. One month after the procedure to induce glaucoma-like conditions, there was a significant average percent loss of RGCs by 28.1% (+/- 5.2, N=20) compared to the internal untreated condition (Figure 1C) in the peripheral retina. In sham studies, sterile PBS was injected into the rat’s episcleral vein instead of 2M NaCl (Figure 1C). There was no significant change in RGC counts associated with injection of PBS or with the procedure of injection by itself.
Neuroprotection of RGCs with a7 nAChR agonists
In this dose-response study, seven commercially available a7 nAChR agonists at three different concentrations were applied as eye drops to the right eyes of adult Long Evans rats that had the procedure to induce glaucoma. The left eyes were not treated and acted as an internal control. One month after the procedure, animals were sacrificed and RGCs were immunostained with antibodies against Thy 1.1, quantified, compared to internal controls and normalized. All a7 nAChR agonists prevented the loss of RGCs in a dose dependent manner (Figure 2). The greatest significant degree of neuroprotection occurred when PHA-543613 was applied as eye drop treatment followed by PNU-282987, nicotine, SEN 12333, tropisetron, 3-bromocytisine and DMAB. Both PHA-543613 and PNU-282987 induced significant RGC neuroprotection when applied as 10 μM. In addition, eye drops containing 100 or 1000 μM PHA-543613 and PNU-282987 provided significantly greater neuroprotection of RGCs compared to any other agent tested. Lastly, when eye drop concentrations of 1 mM were applied, both PHA-543613 and PNU- 282987 eliminated the loss of RGCs associated with the procedure to induce glaucoma-like conditions by an average of 118% +/-10 and 110% +/- 5 respectively. This represented a significant increase of RGCs compared to internal controls (see discussion). In control eye drop studies, application of PBS or PBS in DMSO up to 1% had no significant effect of RGC survival.
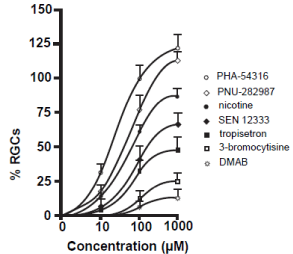
Figure 2: Neuroprotective effect of a7 nAChR agonists.
The line graphs in figure 2 summarize the dose-response experiments
performed using 7 different a7 nAChR agonists. Each data point represents
the average percent of RGCs normalized to internal control numbers obtained
from the same animal and from the same peripheral retinal location. Each
data point was obtained after the right eye of adult Long Evans rats were
treated with various concentrations of the a7 nAChR agonists as eye drops
after the procedure to induce glaucoma-like conditions was performed. The
data points were curve fit. N’s between 4 and 20 were obtained for each
data point. After one month, animals were euthanized, retinas were removed
and RGCs were labeled with anti-Thy 1.1 antibody to label RGCs. Error bars
represent S.E.
Previous studies from this lab using the Tono-Pen XL tonometer (Mentor, Norwell MA) have demonstrated that injection of hypertonic saline into rat episcleral veins significantly increased intraocular pressure [20]. In this current study, the procedure to induce glaucoma-like conditions in adult Long Evans rats significantly increased the average IOP measurement from control untreated levels of 12.8 (+/- 0.5) to 24.3 (+/0 1.2) (Table 1). Topical application (1 mM) of the seven a7 nAChR agonists used in this study for 1 month had no significant effect on the change of IOP measurements (Table 1).
Treatment
Averaged IOP (mm Hg)
(left control eye)
Averaged IOP (mm Hg) (experimental eye after 1 month)
H.I.
11.9 +/- 2.1
22.4 +/- 2.2
H.I. / PHA-543613
12.1 +/- 1.8
24.6 +/- 2.1
H.I./ PNU-282987
10.8 +/- 3/1
23.7 +/- 1/8
H.I./ nicotine
12.1 +/- 2.1
24.2 +/- 3.1
H.I./ SEN 12333
12.6 +/- 1/3
23.6 +/- 3/1
H.I./ tropisetron
10.8 +/- 2.1
21.8 +/- 2.8
H.I./ 3-bromocytisine
11.7 +/- 1.8
22.2 +/- 1.9
H.I./DMAB
10.9 +/- 2.2
21.6 +/- 2/4
Table 1: Mean IOP measurements. H.I. = hypertonic injection into episcleral veins. Each value represents average IOP measurements obtained from 4 different animals.
Figure 3 illustrates the neuroprotective effect of PNU-282987 in the retina. (Figures 3A & 3B) represent typical confocal images of the RGC layer obtained from the same animal and from the same retinal location 4 mm from the ONH. (Figure 3A) was obtained from the untreated retina. In the experimental eye of the same animal (Figure 1B), 1 mM PNU-282987 eye drops were applied before and after the procedure to induce glaucoma-like conditions. One month following the procedure to induce glaucoma, the animal was euthanized, both retinas (treated and untreated) were removed and processed for RGC staining with anti-Thy 1.1 antibody. Anti-Thy 1.1 labeled the plasma membrane of RGC bodies (arrows) as well as bundled RGC axon fascicles (double arrows). When the experimental eye was treated with PNU-282987, there was no evidence of RGC loss typically associated with the procedure to induce glaucoma-like conditions (Figure 3B).
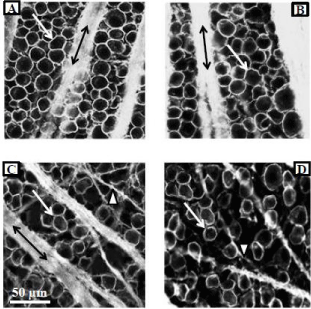
Figure 3: Neuroprotective effect of PNU-282987 is mediated through a7
nAChRs.
Figure 3A represents a typical confocal image obtained from the peripheral
retina in a control untreated adult Long Evans rat. RGCs were immunostained
with anti-Thy 1.1. The left eye of the same animal was treated with 1 mM
PNU-282987 after the procedure to induce glaucoma-like conditions. Figure
3B was obtained from the same retinal location as figure 3A. The typical loss
of RGCs due to the procedure did not occur. The confocal images shown in
3C and D were obtained from another adult Long Evans rat. Retinal images
from both eyes of this animal are shown and both eyes had the procedure
to induce glaucoma-like conditions. However, while the left eye (3C) had
no treatment, the right eye (Figure 3D) had an intravitreal injection of MLA
before the procedure to induce glaucoma-like conditions and subsequent
treatment with PNU-282987. In the presence of MLA, PNU-282987 failed
to provide neuroprotection. Arrows represent RGC bodies, double arrows
represent Thy 1.1 labeled axon fascicles and arrow heads represent axons
that defasciculate off the main axon bundles as RGCs are lost.
In (Figure 3C & 3D), the procedure to induce glaucoma-like conditions was performed on both eyes in the same Long Evans rat. (Figure 3C) illustrates the effect of the procedure on anti-Thy 1.1 immunostained RGCs one month after the procedure. There was a significant loss of RGC bodies from the RGC layer and the bundles RGC axon fascicles (double arrows) begin to defasciculate (arrow head) as axons in the bundle were lost [21]. (Figure 3D) provides evidence that a7 nAChRs are responsible for PNU-282987’s neuroprotective effect. The experimental eye that generated (Figure 3D) was intravitreally injected with 5 μl of 100 nM of the specific a7 nAChR antagonist, MLA, before initiation of PNU-282987 eye drops. In the presence of MLA, the neuroprotective effect of PNU- 28987 was eliminated. These results are summarized in (Figure 4). 1mM PNU-282987 prevented the loss of RGCs typically associated with the procedure to induce glaucoma-like conditions. However, in the presence of the MLA, neuroprotection due to PNU-282987 was reduced in a dose dependent manner. The greatest effect of MLA was demonstrated when 1 mM PNU-282987 eye drop treatments were used after the procedure to induce glaucoma-like conditions with hypertonic saline injections (Figure 4). However, an intravitreal injection of 100 μl MLA into the vitreal chamber before hypertonic saline injections significantly reduced the effect of each a7 nAChR agonist (1 mM eye drop) to varying degrees. MLA was the most effective in reducing PNU-282987’s neuroprotection (Figure 4), followed by PHA-54316, SEN 12333, 3-bromocytisine, tropisetron, DMAB and nicotine.
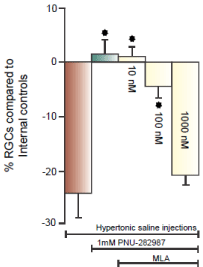
Figure 4: MLA blocks neuroprotection of RGCs.
Each bar graph illustrated in figure 4 represents the average percent of RGC
change compared to internal controls from the same animal after different
treatment conditions including, 1) hypertonic saline injection (brown bar),
2) hypertonic saline injection with PNU-282987 treatment (grey bar) and
3) hypertonic saline injection with PNU-282987 treatment after intravitreal
injections of 3 different concentrations of MLA (yellow bars). Each bar graph
was generated from N’s of 4-20. The star represents significant difference
from the brown bar (hypertonic injection only). Error bars represent S.E.
Discussion
In this study, evidence was provided that several a7 nAChR agonists can be used in the Long Evans rat as a neuroprotective agent against the loss of RGCs normally associated with glaucoma-like conditions. Using a modified version of the Morrison model [24], significant loss of RGCs occurred in an in vivo rat model after injection of hypertonic saline solution into the episcleral vein of the animal’s eye [20,21]. Four weeks after the procedure to induce glaucomalike conditions, RGCs decreased by an average of 28% compared to untreated internal control RGC counts in the periphery of the retina. This model was used to demonstrate the neuroprotective effect of several a7 nAChR agonists in the retina when applied as eye drops.
Intravitreal injections are routinely used to treat retinal conditions such as macular degeneration and macular edema [33-35], However, these injections are invasive and certainly carry some risk. Many stakeholders in drug development would concur topical delivery is a more attractive route for chronic diseases such as glaucoma. As a result, the various a7 nAChR agonists used in this study were applied as eye drops. The main pathway for absorption of eye drops is typically through the cornea. However, it is challenging to obtain therapeutic drug tissue levels in the posterior segment using this route of delivery [36,37].
Two different pieces of evidence support the conclusion that topically applied a7 nAChR agonists used in this study reach the retina: 1) intravitreal MLA blocked the effect of the topically applied agents and 2) previous HPLC MS/MS studies from this lab measured detectable levels of PNU-282987 in the retina after eye drop application using the same treatment procedure as the current study [21]. Treating the retina directly with eye drops has also been used to analyze N-methyl-N-nitrosourea-induced photoreceptor cell death [26], glaucomatous optic neuropathy [27] and to preserve visual function at the retinal ganglion cell layer [25]. Once formulated appropriately for long term use in humans, topical ophthalmic therapy appears to be a non-invasive way to deliver a7 nAChR agonists to the retina.
A growing body of evidence indicates that a7 nAChRs are an attractive target for neuroprotection in the brain and retina. Activation of a7 nAChRs in the brain have been linked to neuroprotection against several neurodegenerative diseases [9,10]. There is strong evidence that a7 nAChRsreduce β-amyloid induced toxicity in animal models of Alzheimer’s disease [11,12] and that the a7 nAChRs plays a role in the pathophysiology of schizophrenia in the brain of humans [13,14]. Previous in vitro studies using adult Long Evans rats have demonstrated that, at certain concentrations, the a7 nAChR agonist, PNU-282987, eliminates RGC loss associated with the procedure to induce glaucoma-like conditions [20,21]. As PNU- 282987 has adverse effects on a hERG anti target in the heart [23], this comparison study was performed to assess the neuroprotective effect of other a7 nAChR agonists, some of which may have a better product safety profile.
PHA-543613 was found to have the largest neuroprotective effect on RGC survival after the procedure to induce glaucoma-like conditions. Like PNU-282987, 1 mM PHA-543613 eliminated the loss of RGCs associated with the procedure. In fact, both PHA-543613 and PNU-282987 resulted in significantly more RGCs compared to their internal controls when 1 mM was applied as eye drops. This data suggests that neuroprotection of RGCs in a mammalian system may be possible when relatively high concentrations of a7 nAChR agonists are used.
PHA-543613 is a furo pyridine proved to be a potent, high affinity agonist of alpha7 nAChRs under development for the therapeutic treatment of cognitive disorders in people with schizophrenia and Alzheimer’s disease [38,39]. PHA-543613 has been shown to be active in relevant models in both in vitro and in vivo studies [38,39]. It demonstrates high oral bioavailability in rats and has a more favorable hERG profile than PNU-282987 [39]. It rapidly penetrates the blood brain barrier and shows effective auditory sensory gating and objects recognition in an in vivo rat model [39], enhances the neurovascular response in an Alzheimer’s mouse model to improve recognition memory [40] and protects dopaminergic neurons in a rat Parkinson’s model [41].
All of the other agents applied to adult Long Evans rat eyes provided neuroprotection to a lesser degree than PHA-543613 and PNU-282987. This inferiority is likely due to the less robust activity at the target receptor as well as action of the agents at other receptors. Tropisetron, 3-Bromocytisine, SEN 12333 and DMAB are classified as partial a7 agonists with affinities for other types of receptors. Tropisetron is a potent 5-HT3 antagonist and partial agonist of a7 nAChRs [42-44]. SEN 12333 has been found to be an a7 nAChR agonist as well as an antagonist for H3 receptors and shows weak agonist activity at a3 nAChRs [45,46]. 3-Bromocytisine is a nicotinic agonist of many different functional ACh receptors including a4β4, a4β2 and a7 nAChRs [47,48], while DMAB has been used as a partial agonist at a7 nAChRs to improve learning and memory in rats but is also an a4β2 antagonist [49-51].
The results using PHA-543613 and PNU-282987, as well as the selective a7 nAChR antagonist, MLA, support the hypothesis that activation of a7 nAChRs represents a path towards neuroprotection in the retina. All currently available treatments for glaucoma are currently focused on reducing IOP, the primary risk factor associated with glaucoma [30-32]. These topical pharmaceutical glaucoma therapies decrease the production of aqueous humor or alter outflow fluid dynamics. Surgical procedures are similarly predominant technologies to enhance the drainage of aqueous humor [52]. However, these treatments alone are insufficient to halt the progression of blindness associated with glaucoma [53-55]. New treatment approaches are needed to prevent the loss of RGCs in glaucoma and a7 nAChR agonists may someday become an effective intervention in this setting.
How could activation of a7 nAChRs in the retina induce neuroprotection to prevent the loss of RGCs associated with glaucoma? In previous studies using isolated porcine and rat RGCs, ELISA and immunocytochemical studies have demonstrated that ACh activation of a7 nAChRs triggered activation of the PI3→Akt→Bcl2 signaling cascade to provide neuroprotection against glutamate induced excitotoxicity [56,57]. These studies hypothesized that calcium influx through ionotropic a7 nAChR channels was involved in triggering the intracellular cascade to induce neuroprotection. Other studies have demonstrated that the link between glutamate receptor activation and signaling cascade initiation is calcium permeation through glutamate channels [58,59]. It may be that permeation of extracellular calcium through nAChR channels is the key to inducing neuroprotective pathways in rat RGCs in glaucoma. In this scenario, calcium permeation through a7 nAChRs triggers neuroprotective signaling cascades or may induce neuroprotection through other mechanisms, such as internalization of NMDA receptors [60].
Conclusion
The results from this paper demonstrate the neuroprotective effect of several alpha7 nAChR agonists on RGC survival in a rat glaucoma model. Each agonist provided neuroprotection in a dose dependent manner although the largest neuroprotective effects were produced when PHA-543613 and PNU-282987 were applied topically. The results from this study have also provided evidence that eye drop application of the agonists is sufficient to prevent loss of RGCs in the Long Evans rat. These results support a scenario where future therapies for neurodegenerative diseases of the CNS may involve, either alone or in combination with other agents, the pharmacologic activation of alpha7 nAChRs.
References
- Gordon MO, Beiser JA, Brandt JD, Heuer DK, Higginbotham EJ, Johnson CA, et al. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 714-720.
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004; 82: 844-851.
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006; 90: 262-267.
- Medeiros FA, Alencar LM, Zangwill LM, Bowd C, Sample PA, Weinreb RN. Prediction of functional loss in glaucoma from progressive optic disc damage. Arch Ophthalmol. 2009; 127: 1250-1256.
- Fraser CL, White AJ, Plant GT, Martin KR. Optic nerve cupping and the neuro-ophthalmologist. J Neuroophthalmol. 2013; 33: 377-389.
- Guo L, Moss SE, Alexander RA, Ali RR, Fitzke FW, Cordeiro MF. Retinal ganglion cell apoptosis in glaucoma is related to intraocular pressure and IOP-induced effects on extracellular matrix. Invest Ophthalmol Vis Sci. 2005; 46: 175-182.
- Fu QL, Li X, Shi J, Xu G, Wen W, Lee DH, et al. Synaptic degeneration of retinal ganglion cells in a rat ocular hypertension glaucoma model. Cell Mol Neurobiol. 2009; 29: 575-581.
- Jha P, Banda H, Tytarenko R, Bora PS, Bora NS. Complement mediated apoptosis leads to the loss of retinal ganglion cells in animal model of glaucoma. Mol Immunol. 2011; 48: 2151-2158.
- Conejero-Goldberg C, Davies P, Ulloa L. Alpha7 nicotinic acetylcholine receptor: a link between inflammation and neurodegeneration. Neurosci Biobehav Rev. 2008; 32: 693-706.
- Liu Z, Cai H, Zhang P, Li H, Liu H, Li Z. Activation of ERK1/2 and PI3K/Akt by IGF-1 on GAP-43 expression in DRG neurons with excitotoxicity induced by glutamate in vitro. Cell Mol Neurobiol. 2012; 32: 191-200.
- Kawamata J, Shimohama S. Stimulating nicotinic receptors trigger multiple pathways attenuating cytotoxicity in models of Alzheimer's and Parkinson's diseases. J Alzheimers Dis. 2011; 24: 95-109.
- Oz M, Lorke DE, Yang KH, Petroianu G. On the interaction of β-amyloid peptides and a7-nicotinic acetylcholine receptors in Alzheimer's disease. Curr Alzheimer Res. 2013; 10: 618-630.
- Winterer G, Gallinat J, Brinkmeyer J, Musso F, Kornhuber J, Thuerauf N, et al. Allosteric alpha-7 nicotinic receptor modulation and P50 sensory gating in schizophrenia: a proof-of-mechanism study. Neuropharmacology. 2013; 64: 197-204.
- Young JW, Geyer MA. Evaluating the role of the alpha-7 nicotinic acetylcholine receptor in the pathophysiology and treatment of schizophrenia. Biochem Pharmacol. 2013; 86: 1122-1132.
- Whiting PJ, Schoepfer R, Conroy WG, Gore MJ, Keyser KT, Shimasaki S, et al. Expression of nicotinic acetylcholine receptor subtypes in brain and retina. Brain Res Mol Brain Res. 1991; 10: 61-70.
- Keyser KT, Britto LR, Schoepfer R, Whiting P, Cooper J, Conroy W, et al. Three subtypes of alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors are expressed in chick retina. J Neurosci. 1993; 13: 442-454.
- Kaneda M, Hashimoto M, Kaneko A. Neuronal nicotinic acetylcholine receptors of ganglion cells in the cat retina. Jpn J Physiol. 1995; 45: 491-508.
- Masland RH, Mills JW, Hayden SA. Acetylcholine-synthesizing amacrine cells: identification and selective staining by using radioautography and fluorescent markers. Proc Roy Soc Lond Biol Sci. 1984; 223: 79-100.
- Massey SC, Redburn DA. Transmitter circuits in the vertebrate retina. Prog Neurobiol. 1987; 28: 55-96.
- Iwamoto K, Birkholz P, Schipper A, Mata D, Linn DM, Linn CL. A nicotinic acetylcholine receptor agonist prevents loss of retinal ganglion cells in a glaucoma model. Invest Ophthalmol Vis Sci. 2014; 55: 1078-1087.
- Mata D, Linn DM, Linn CL. Retinal ganglion cell neuroprotection induced by activation of alpha7 nicotinic acetylcholine receptors. Neuropharmacology. 2015; 99: 337-346.
- Bodnar AL, Cortes-Burgos LA, Cook KK, Dinh DM, Groppi VE, Hajos M, et al. Discovery and structure-activity relationship of quinuclidinebenzamides as agonists of alpha7 nicotinic acetylcholine receptors. J Med Chem. 2005; 48:905-908.
- Walker DP, Wishka DG, Piotrowski DW, Jia S, Reitz SC, Yates KM, et al. Design, synthesis, structure-activity relationship, and in vivo activity of azabicyclic aryl amides as alpha7 nicotinic acetylcholine receptor agonists. Bioorg Med Chem. 2006; 14: 8219-8248.
- Morrison JC, Moore CG, Deppmeier LM, Gold BG, Meshul CK, Johnson EC. A rat model of chronic pressure-induced optic nerve damage. Exp Eye Res. 1997; 64: 85-96.
- Prokai-Tatrai K, Xin H, Nguyen V, Szarka S, Blazics B, Prokai L, et al. 17β-estradiol eye drops protect the retinal ganglion cell layer and preserve visual function in an in vivo model of glaucoma. Mol Pharm. 2013; 10: 3253-3261.
- Lin JL, Wang YD, Ma Y, Zhong CM, Zhu MR, Chen WP, et al. Protective effects of naringenin eye drops on N-methyl-N-nitrosourea-induced photoreceptor cell death in rats. Int J Ophthalmol. 2014; 7: 391-396.
- Roberti G, Tanga L, Parisi V, Sampalmieri M, Centofanti M, Manni G. A preliminary study of the neuroprotective role of citicoline eye drops in glaucomatous optic neuropathy. Indian J Ophthalmol. 2014; 62: 549-553.
- Barnstable CJ, Dräger UC. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984; 11: 847-855.
- Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, et al. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995; 15: 4762-4785.
- Chauhan BC, Pan J, Archibald ML, Le Vatte TL, Kelly ME, Tremblay F. Effect of intraocular pressure on optic disc topography, electroretinography, and axonal loss in a chronic pressure-induced rat model of optic nerve damage. Invest Ophthalmol Vis Sci. 2002; 43: 2969-2976.
- Levkovitch-Verbin H, Quigley HA, Martin KR, Valenta D, Baumrind LA, Pease ME. Translimbal laser photocoagulation to the trabecular meshwork as a model of glaucoma in rats. Invest Ophthalmol Vis Sci. 2002; 43: 402-410.
- Damji KF, Behki R, Wang L. Target IOP Workshop participants. Canadian perspectives in glaucoma management: setting target intraocular pressure range. Can J Ophthalmol. 2003; 38: 189-197.
- Kernt M, Cserhati S, Seidensticker F, Liegl R, Kampik A, Neubauer A, et al. Improvement of diabetic retinopathy with intravitreal Ranibizumab. Diabetes Res Clin Pract. 2013; 100: 11-13.
- Song WT, Xia XB. Ranibizumab for macular edema secondary to retinal vein occlusion: a meta-analysis of dose effects and comparison with no anti-VEGF treatment. BMC Ophthalmol. 2015; 15: 31.
- Batioglu F, Demirel S, Özmert E, Abdullayev A, Bilici S. Short-term outcomes of switching anti-VEGF agents in eyes with treatment-resistant wet AMD. BMC Ophthalmol. 2015; 15: 40.
- Ahmed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci. 1985; 26: 584-587.
- Geroski DH, Edelhauser HF. Drug delivery for posterior segment eye disease. Invest Ophthalmol Vis Sci. 2000; 41: 961-964.
- Acker BA, Jacobsen EJ, Rogers BN, Wishka DG, Reitz SC, Piotrowski DW, et al. Discovery of N-[(3R,5R)-1-azabicyclo[3.2.1]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide as an agonist of the alpha7 nicotinic acetylcholine receptor: in vitro and in vivo activity. BioorgMed Chem Lett. 2008; 18: 3611-3615.
- Wishka DG, Walker DP, Yates KM, Reitz SC, Jia S, Myers JK, et al. Discovery of N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]furo[2,3-c]pyridine-5-carboxamide, an agonist of the alpha7 nicotinic acetylcholine receptor, for the potential treatment of cognitive deficits in schizophrenia: synthesis and structure--activity relationship. J Med Chem. 2006; 49: 4425-4436.
- Sadigh-Eteghad S, Mahmoudi J, Babri S, Talebi M. Effect of alpha-7 nicotinic acetylcholine receptor activation on beta-amyloid induced recognition memory impairment. Possible role of neurovascular function. Acta Circ Bras. 2015; 30: 736-742.
- Sérrière S, Doméné A, Vercouillie J, Mothes C, Bodard S, Rodrigues N, et al. Assessment of the Protection of Dopaminergic Neurons by an a7 Nicotinic Receptor Agonist, PHA 543613 Using [(18)F]LBT-999 in a Parkinson's Disease Rat Model. Front Med (Lausanne). 2015; 2: 61.
- Macor JE, Gurley D, Lanthorn T, Loch J, Mack RA, Mullen G, et al. The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett. 2001; 11: 319-321.
- Papke RL, Schiff HC, Jack BA, Horenstein NA. Molecular dissection of tropisetron, an alpha7 nicotinic acetylcholine receptor-selective partial agonist. Neurosci Lett. 2005; 378: 140-144.
- Swartz MM, Linn DM, Linn CL. Tropisetron as a neuroprotective agent against glutamate-induced excitotoxicity and mechanisms of action. Neuropharmacology. 2013; 73: 111-121.
- Roncarati R, Scali C, Comery TA, Grauer SM, Aschmi S, Bothmann H, et al. Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. J Pharmacol Exp Ther. 2009; 329: 459-468.
- Beinat C, Banister SD, Van Prehn S, Doddareddy MR, Hibbs D, Sako M, et al. Consequences of linker length alteration of the a7 nicotinic acetylcholine receptor (nAChR) agonist, SEN12333. Bioorg Med Chem Lett. 2012; 22: 2380-2384.
- Houlihan LM, Slater Y, Guerra DL, Peng JH, Kuo YP, Lukas RJ, et al. Activity of cytisine and its brominated isosteres on recombinant human alpha7, alpha4beta2 and alpha4beta4 nicotinic acetylcholine receptors. J Neurochem. 2001; 78: 1029-1043.
- Moroni M, Zwart R, Sher E, Cassels BK, Bermudez I. alpha4beta2 nicotinic receptors with high and low acetylcholine sensitivity: pharmacology, stoichiometry, and sensitivity to long-term exposure to nicotine. Mol Pharmacol. 2006; 70: 755-768.
- Arendash GW, Sengstock GJ, Sanberg PR, Kem WR. Improved learning and memory in aged rats with chronic administration of the nicotinic receptor agonist GTS-21. Brain Res. 1995; 674: 252-259.
- Kem WR, Mahnir VM, Papke RL, Lingle CJ. Anabaseine is a potent agonist on muscle and neuronal alpha-bungarotoxin-sensitive nicotinic receptors. J Pharmacol Exp Ther. 1997; 283: 979-992.
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl). 1998; 136: 320-327.
- Cairns JE. Trabeculectomy. Preliminary report of a new method. Am J Ophthalmol. 1968; 66: 673-679.
- Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002; 120: 1268-1279.
- Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002; 120: 701-713.
- Beidoe G, Mousa SA. Current primary open-angle glaucoma treatments and future directions. Clin Ophthalmol. 2012; 6: 1699-1707.
- Asomugha CO, Linn DM, Linn CL. ACh receptors link two signaling pathways to neuroprotection against glutamate-induced excitotoxicity in isolated RGCs. J Neurochem. 2010; 112: 214-226.
- Iwamoto K, Mata D, Linn DM, Linn CL. Neuroprotection of rat retinal ganglion cells mediated through alpha7 nicotinic acetylcholine receptors. Neuroscience. 2013; 237: 184-198.
- Lam TT, Abler AS, Kwong JM, Tso MO. N-methyl-D-aspartate (NMDA)--induced apoptosis in rat retina. Invest Ophthalmol Vis Sci. 1999; 40: 2391-2397.
- Quigley HA. Neuronal death in glaucoma. Prog Retin Eye Res. 1999; 18: 39-57.
- Akopian A, Szikra T, Cristofanilli M, Krizaj D. Glutamate-induced Ca2+ influx in third-order neurons of salamander retina is regulated by the actin cytoskeleton. Neuroscience. 2006; 138: 17-24.