
Research Article
J Ophthalmol & Vis Sci. 2020; 5(2): 1041.
The Role of Spironolactone and Rifampicin in the Management of Chronic Central Serous Chorioretinopathy - A Prospective Study
Agarwal M¹*, Ranjan R¹, Gujral GS¹, Chowdhary N¹, Shrivastav A¹, Kumar S¹, Mayor R¹, Paul L¹, Singh S¹ and Trehan HS²
¹Dr. Shroff’s Charity Eye Hospital, India
²Department of Ophthalmology, Army Hospital (Research and Referral), India
*Corresponding author: Agarwal M, Vitreoretina Services, Dr. Shroff’s Charity Eye Hospital, 5027-Kedar Nath Road, Daryaganj, New Delhi, India
Received: November 23, 2020; Accepted: December 15, 2020; Published: December 22, 2020
Abstract
Aim: To evaluate the efficacy and safety of spironolactone and rifampicin in the management of chronic Central Serous Chorioretinopathy (CSCR).
Result: 19 eyes (16 patients) in group-1, 22 eyes (18 patients) in group-2 and 21 eyes (15 patients) in group-3. The primary end point was achieved in 6/19 eyes, 5/22 eyes and 5/21 eyes in group-1, 2 and 3 respectively. Best Corrected Visual Acuity (BCVA) using log MAR charts showed a statistically significant change at 2 months to 0.22±0.24 (p-value 0.028), 0.19±0.22 (p-value 0.027) and 0.23±0.28 (p-value 0.030) in group-1, 2 and 3 respectively. The recurrence of SRF at 6 months was noted in 25%, 62.5% and 69.23% of eyes in group-1, 2 and 3 respectively. None of the patients showed any side effect of the drugs.
Conclusion: Spironolactone and placebo were comparable in terms of resolution of the SRF at the 2 months. Placebo had least number of recurrences at 6months in comparison to both spironolactone and rifampicin, thereby making placebo (antioxidants) a better choice for the treatment of chronic CSCR. However, studies with a larger sample size are required to establish the role of these drugs in the management of chronic CSCR.
Keywords: CSCR; Spironolactone; Rifampicin; Anti-oxidant
Abbreviations
CSCR: Central Serous Chorioretinopathy; SRF: Subretinal Fluid; RPE: Retinal Pigment Epithelial; PDT: Photodynamic Therapy; Anti- VEGF: Anti-Vascular Endothelial Growth Factor; SD: Standard Deviation; LFT: Liver Function Test; RFT: Renal Function Test; TB: Tuberculosis; BP: Blood Pressure; K+: Potassium; Na+: Sodium; FFA: Fundus Fluorescein Angiography; BCVA: Best Corrected Visual Acuity; OCT: Optical Coherence Tomography; CBC: Complete Blood Count; ATT: Anti-Tubercular Treatment; NNT: Number Needed to Treat; NNH: Number Needed to Harm
Introduction
Central Serous Chorioretinopathy (CSCR) is a self-limiting disease with neurosensory retinal detachment at the posterior pole with often good visual recovery [1,2]. Most common in men aged 30 to 50 years. Chronic CSCR is defined as Subretinal Fluid (SRF) more than 3months. Retinal Pigment Epithelial (RPE) changes also signify chronicity [3,4]. The exact pathogenesis of CSCR is unclear. Increased levels of endogenous (Cushing disease, pregnancy and stress) and exogenous corticosteroids are said to be a risk factor along with the vasodilatation of choroidal vessels. The main modality of treatment for chronic CSCR with sub-foveal leaks is Photodynamic Therapy (PDT) [5,6] and micropulse laser [7], for extrafoveal leaksfocal laser photocoagulation [8]. Various pharmacotherapeutic agents used are-spironolactone [3,9-12], rifampicin [4,13,14], ketoconazole [15], mifepristone [16], eplerenone [17-19], antioxidants [20] and intravitreal anti-Vascular Endothelial Growth Factor (anti-VEGF) [21,22]. Anecdotal case reports have shown spironolactone and rifampicin to be efficacious in chronic CSCR. As these drugs were affordable and accessible by our patients, we thus chose them for our prospective study and evaluated their role in comparison to a placebo (antioxidant) in managing chronic CSCR patients.
Methods
A prospective, randomized, placebo-controlled crossover study was conducted adhering to the Tenets of the declaration of Helsinski, after obtaining an informed consent and an approval from the Institutional Ethics committee (SCEH-2012-04-003). 51 eyes of 41 patients with chronic CSCR defined as persistent SRF>3 months were included. All the patients of chronic CSCR between March 2016 to December 2018 at a tertiary eye care centre of North India were included. Based on a previous study which showed the percentage change in SRF thickness after spironolactone was 38.2% and after placebo was 0.8% [9]. Taking these as reference and assuming Standard Deviation (SD) of 30, power of study-80% and 5% level of significance, 11 patients in each study group were required.
The inclusion criteria were: patients>18 years of age with chronic idiopathic CSCR not amendable to focal laser, no co-existent retinal pathology, normal Liver Function Test (LFT) and Renal Function Test (RFT), no history of Tuberculosis (TB) or contact, Normal Blood Pressure (BP), Potassium (K+) and Sodium Levels (Na+), no history of corticosteroid intake within 3months. The exclusion criteria were: patients <18 years, not giving informed consent, persistent SRF<3 months, focal leakage on Fundus Fluorescein Angiography (FFA) amenable to laser, co-existent retinal pathology, abnormal LFT or RFT, positive history of TB, uncontrolled BP, deranged K+/Na+ levels and intake of corticosteroids within 3 months.
A comprehensive ophthalmologic examination including Best Corrected Visual Acuity (BCVA) taken as log MAR charts, indirect ophthalmoscopy, FFA at baseline and Optical Coherence Tomography (OCT; Cirrus HD-OCT; Carl Zeiss Meditec) at every visit was done. A built-in caliper scale was used to measure SRF height (between the outer segment line and the RPE layer at the foveal centre) and horizontal length, sub-foveal retinal and choroid thickness (between RPE layer and inner surface of the sclera) (Figure 1). Two independent blinded observers measured the OCT parameters and the mean value was taken for analysis. The chosen section was kept constant in all the follow-up visits. Baseline evaluation of LFT and RFT, Complete Blood Count (CBC), serum cortisol levels, Mantoux test and BP measurement was done. Treatment success was defined as resolution of the SRF<2 months after the initiation of treatment. Treatment failure was defined as non-resolution of the SRF after 2 months of initiating treatment.
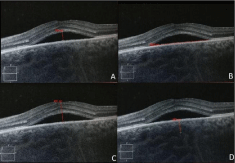
Figure 1: Showing various OCT parameters measurement. A-showing SRF
height, B showing horizontal SRF length, C-retinal thickness, D-choroidal
thickness.
Computer-generated random permuted blocks was used to randomize patients in the following 3 groups:
Group-1: Placebo group (antioxidant capsule once a day)
Group-2: Rifampicin-600mg once daily
Group-3: Spironolactone-50mg once daily
The drug was continued for a maximum period of two months. Follow up was done at 1, 2, 3 and 6 months after initiating a drug. In case of non-resolution of SRF after a drug for 2months, the patients were given an option of crossover to another drug after a wash off period of 1month. The patient was then started on another drug for a maximum of 2 months. A patient after crossover to another group was considered as a new patient for that group. Primary end point was complete resolution of SRF within 2 months of a drug. Secondary end points were (1) Change in the BCVA, (2) Number of recurrences, (3) Side effects of the drug.
SPSS software (version 24; IBM Inc., Chicago, IL, USA) was used for statistical analysis. Paired t-test was used to analyse within group changes from baseline. Results were reported as mean± SD. P-values<0.05 was considered statistically significant.
Results
Initially there were 16 eyes of 13 patients in the placebo group, 19 eyes of 16 patients in the rifampicin group and 16 eyes of 12 patients in the spironolactone group. However, after the cross over there were 19 eyes of 16 patients in the placebo group, 22 eyes of 18 patients in the rifampicin group and 21 eyes of 15 patients in the spironolactone group. The mean age was 37.62±9.85 years (range 25- 58 years) in group-1, 41.17±8.09 years (range 23-55 years) in group-2 and 37.8±5.8 years (range 24-45 years) in group-3 (Table 1). The mean duration of SRF was 9.38±9.86 months (range 3-36 months) in group-1, 9.39±9.27 months (range 3-36 months) in group-2, 9.38±12.87 months (range 3-48 months) in group-3.
Group 1
Placebo
(16 patients)
Group 2
Rifampicin
(18 patients)
Group 3
Spironolactone
(15 patients)
P-value
Gender distribution (M:F)
15:1
16:2
14:1
0.847
Age distribution
1) <=30
6
2
2
0.056
2) 31-40
5
5
10
3) 41-50
3
9
3
4) >50
2
2
0
Laterality
Bilateral
3
4
6
0.081
Unilateral
13
14
9
Table 1: Demographic Profile of the patients.
The placebo group showed a statistically significant BCVA improvement was noted at 2 months (p-value 0.028) as compared to baseline, which further improved at 3 months (p-value 0.005). 6/19 eyes (31.58%) showed a complete resolution of SRF at 2 months. A statistically significant reduction of SRF height was seen at 1 and 2 months (p-value 0.005 and 0.043 respectively) of follow up and statistically significant reduction of the horizontal SRF length was noted at 2 and 3 months (p-value 0.035 and 0.025 respectively) of follow up compared to the baseline values. The choroidal thickness showed a statistically significant reduction (p-value 0.034 and 0.024) at 1 and 2 months (Table 2). The rifampicin group showed the BCVA improvement was statistically significant at 2 months (p-value-0.027). 5/22 eyes (22.73%) showed a complete resolution of SRF at 2 months. There was no statistically significant reduction in SRF height (p-value 0.085) and horizontal SRF length (p-value 0.151) at 2months compared to baseline. There was a statistically significant difference in the choroidal thickness between baseline and at 2 months (p-value 0.003) (Table 3). The spironolactone group showed the BCVA improvement was statistically significant at 2 months (p-value 0.030) which remained so at 3 months (p-value 0.001). 5/21 eyes (23.81%) showed a complete resolution of SRF at 2 months. There was a statistically significant reduction in the height of SRF (p-value 0.016) and horizontal SRF length (p-value 0.000) at 2 months. No statistically significant reduction was seen in the choroidal thickness (p-value 0.107) (Table 4). The primary end point of complete resolution of the SRF within 2 months of starting the drug was achieved in 6/19 eyes, 5/22 eyes and 5/21eyes in group-1, 2 and 3 respectively. On follow up at 3 months, complete resolution of SRF was found in 8/19 eyes, 8/22 eyes and 13/21 eyes in group-1, 2 and 3 respectively. Therefore maximum eyes in spironolactone group showed complete resolution of SRF at 3 months. A recurrence of SRF within 6 months was seen in 2/8 eyes (25%), 5/8 eyes (62.5%) and 9/13 eyes (69.23%) in group-1, 2 and 3 respectively. Therefore, eyes in spironolactone group showed maximum recurrence (Table 5). None of the patients showed any side effect of the drugs used.
Baseline (sample size 19)
1 month (sample size 19)
2 months (sample size 19)
3 months (sample size 19)
BCVA
0.31±0.27
0.23±0.26
0.22±0.24
0.18±0.21
P-value
0.017
0.028
0.005
SRF height
132.16±97.61
71.26±54.15
74.42±74.98
74.47±86.17
P-value
0.005
0.043
0.073
SRF horizontal length
2261±1088.6
1827±1398.67
1494.1±1383.52
1280.37±1349.74
P-value
0.089
0.035
0.025
Retina thickness
301.1±129.67
251.58±85.83
260.53±105.33
267.21±87.55
P-value
0.024
0.157
0.284
Choroid thickness
393.05±103.88
370.1±103.16
370.63±105.22
361.68±76.89
P-value
0.034
0.024
0.187
Table 2: Baseline and follow up parameters in the placebo group.
Baseline (sample size 22)
1 month (sample size 22)
2 months (sample size 22)
3 months (sample size 22)
BCVA
0.29±0.26
0.21±0.24
0.19±0.22
0.26±0.28
P-value
0.051
0.027
0.660
SRF height
121.14±87.69
104.77±86.1
82.86±70.48
72.04±82.85
P-value
0.417
0.085
0.037
SRF horizontal length
1999.64±1172.91
1736.41±1222.54
1625.77±1381.97
1413±1606.25
P-value
0.262
0.151
0.056
Retina thickness
253±98.64
255.14±100.88
239.18±85.94
237.18±90.54
P-value
0.890
0.465
0.385
Choroid thickness
385.73±93.04
365.96±109.96
351.64±107.94
366.54±91.7
P-value
0.025
0.003
0.024
Table 3: Baseline and follow up parameters in the rifampicin group.
Baseline
(sample size 21)
1 month (sample size 21)
2 months (sample size 21)
3 months (sample size 21)
BCVA
0.31±0.27
0.26±0.28
0.23±0.28
0.19±0.24
P-value
0.179
0.030
0.001
SRF height
131.43±92.32
80.43±58.74
65.57±55.86
45.38±71.11
P-value
0.011
0.016
0.008
SRF horizontal length
2826.52±1578.95
1513.19±1035.23
1331.86±1173.05
672.48±1001.59
P-value
0.001
0.000
0.000
Retina thickness
284.95±103.95
234.1±79.9
225.14±67.86
194.95±72.67
P-value
0.009
0.022
0.003
Choroid thickness
348.95±72.78
354.14±86.21
326.95±90.43
328.52±95.3
P-value
0.448
0.107
0.227
Table 4: Baseline and follow up parameters in the spironolactone group.
Placebo
Rifampicin
Spironolactone
Complete Resolution of SRF
1 month
4/19
(21.05%)
(CI-0.86%-41.24%)
3/22
(13.64%)
(CI-1.94%-29.21%)
3/21
(14.28%)
(CI-2.04%-30.61%)
2 months
6/19
(31.58%)
(CI- 8.56%-54.60%)
5/22
(22.73%)
(CI-3.71%-41.75%)
5/21
(23.81%)
(CI-3.94%-43.68%)
3 months
8/19
(42.10%)
(CI-17.66%-66.55%)
8/22
(36.36%)
(CI-14.53%-58.19%)
13/21
(61.90%)
(CI-39.25%-84.56%)
Number of eyes with recurrence of SRF
6 months
2/8
(25%)
(CI-13.70%-63.70%)
5/8
(62.5%)
(CI-19.23%-100.00%)
9/13
(69.23%)
(CI-40.20%-98.26%)
CI- 95% Confidence Interval.
Table 5: Number of eyes with complete resolution and recurrence of SRF.
Discussion
CSCR patients complain of blurring of vision and seeing a central scotoma secondary to a neurosensory detachment at the macula [1]. It is often self-limiting but in 10-20 % it may become chronic with persistence of SRF leading to photoreceptor loss and extensive RPE damage and choroidal neovascularization. This may cause permanent damage to BCVA, color vision and contrast sensitivity [23]. Currently, no consensus exists regarding the treatment of chronic CSCR and novel treatments are being explored. The exact pathogenesis of the disease is unknown. A forme fruste of CSCR forms a part of the pachychoroid spectrum known as pachychoroid pigment epitheliopathy. The possible mechanisms include increased capillary fragility leading to choroidal circulation decompensation with leakage of the fluid in the sub-retinal space [24]. Glucocorticoids are implicated in the pathogenesis of CSCR and effect vascular autoregulation [25]. Rifampicin is an anti-bacterial drug and form a part of the Anti-Tubercular Treatment (ATT). It is a cytochrome P450 3A4 enzyme inducer, which increases the metabolism of endogenous corticosteroids and thereby reduces their levels in the serum, thereby helping in faster SRF resolution. It is said to have an anti-oxidative, anti-apoptotic and anti-angiogenic effects [13]. On review of literature, the first report on the effect of rifampicin in chronic CSCR was described by Ravage and Packo in 2010 [26]. They reported one patient having concomitant chronic CSCR and tuberculosis receiving rifampicin 600 mg/day as part of the ATT. There was significant improvement in symptoms, which recurred on stopping the drug and improved on reinstitution of the drug establishing a causal relationship. The largest case series of 22 patients by Sabouri et al., [27] have reported the use of rifampicin-600 mg/ day for 4-6 weeks in acute CSCR of less than 2 weeks. The mean age was 38.5±6.7 years and there was a statistically significant reduction in the macular thickness (p<0.003). In 45.5%, there was complete resolution of the SRF in comparison to 29.4% in the control group however, this was not statistically significant. There was no relapse on 9 months follow up.1 patient had severe headache 2 weeks after starting the drug.
Choudhury and co-workers [28] treated 13 CSCR patients with a duration less than 6 weeks. 10 patients showed improvement in vision with a decrease in CMT after rifampicin 600 mg/day for 4 weeks. However, the duration of the follow up was not defined with no mention of recurrence. Shulman et al., [13] in their series of 14 eyes (12 patients) with a mean duration of 28. 4 months and not treatment naive were treated for 3 months with rifampicin 300mg twice daily. There was a statistically significant reduction (p<0.05) in the retinal thickness by 25.3%, 21.2% and 21% at 1, 2 and 3 months respectively. SRF had reduced in 9 eyes (64%) and completely resolved in 6 eyes (42.8%) at month 3, of which 4 eyes remained fluid free at 6 months. There was a statistical significant improvement in BCVA (p>0.05). Two patients stopped the treatment after 2 months due to increase in blood pressure as rifampicin increases the metabolism of calcium channel blockers and the second patient developed acute cholecystitis as rifampicin is thought to increase bile acid production. Study by Venkatesh R et al., [4] 9 eyes (8 patients) with an average duration of 16 months (3-60 months). 4 eyes had sub-foveal leaks and 5 eyes had diffuse retinal pigment epitheliopathy. Treated with rifampicin- 600 mg/day for maximum period of 3 months. Complete resolution of SRF was achieved in 4/9 eyes with 2 patients at 1month and 1patient each at 2 and 3months. BCVA improvement was noted in 4/9 eyes. Mahar PS et al., [14] reported the use of Rifampin-450 mg/day in 10 patients of chronic CSCR (>6 months) with an average duration of SRF as 9.4+2.9 months. An improvement in the macular thickness was noted at 3 months from 350+82.3 to 232+54.3 μm.
The optimal dosage and duration of rifampicin in treating chronic CSCR is still unknown. In our study all the patients of chronic CSCR with a mean duration of SRF as 9.39+9.27 months. Our patients received 600mg once daily for a maximum of 2 months. 5/22 eyes showed a complete resolution of the SRF at 2 months which became 8 eyes at 3 months. However the reduction in the SRF height (p=0.085) and horizontal length of SRF (p=0.151) was not statistically significant. The BCVA improvement at 2 months in comparison to baseline was found to be statistically significant (p=0.027). Recurrence was seen in 5/8 (62.5%) eyes at 6 months. No side effect of the drug was noted. We need to keep in mind that rifampicin being hepatotoxic and having several drug interactions, a detailed medical history and LFTs at baseline are mandatory before starting the drug. The glucocorticoidinduced effects may result in choroid vessel dilatation and leakage, effects which are reversible with MR antagonists. The molecular target for MR activation in the choroid is namely KCa2.3 which is an endothelial hyperpolarizing calcium dependent potassium channel involved in vasorelaxation and inducing dilation of choroidal vessels [25]. Any mineralocorticoid receptor antagonist including spironolactone or eplerenone reverses the upregulation of KCa2.3 in the choroid demonstrating their local molecular action.
Spironolactone is the oldest MR antagonist which may exert hormonal effects at a high dose and prolonged use (>3 months) including reduced libido, gynecomastia and menstrual disturbance [29]. Eplerenone is more specific with minimal hormonal effects but has a 50 fold reduced efficacy on MR blockage and is more expensive [30]. We used spironolactone as it is more economical, more efficacious and the duration of use was short therefore the possibility of hormonal side effects was minimal. Bousquet et al., [9] in their prospective, randomized, placebo controlled crossover study of 16 eyes of 16 patients with a mean duration of SRF as 10+16.9 months. Patients were randomized to receive spironolactone 50mg or placebo once a day for 30 days. A crossover was done with a washout period of 1 week. Crossover data analysis showed a statistically significant reduction in SRF in the spironolactone group as compared to the placebo group with a significant reduction in the sub-foveal choroidal thickness in the treated eyes (p=0.02).No significant changes were noted in the visual acuity and no complications. Kapoor et al., [3] reported the role of spironolactone in the management of CSCR secondary to 3 recent steroid injections given in the back of a Caucasian female. There was evidence of pachychoroid in both the eyes. SRF showed regression on using spironolactone 50mg twice daily, increased to 50mg thrice daily for another month resulting in complete resolution of the SRF. There are several isolated case reports describing the use of spironolactone [3] and epilerenone [17,18]. In our study in the spironolactone group, the mean duration of SRF was 9.38+12.87 months. Our patients received spironolactone 50mg once daily for a maximum of 2months. 5/21 eyes showed a complete resolution of the SRF at 2months which increased to 13 eyes at 3 months. However the reduction in the height of SRF (p=0.016, p=0.008) and horizontal length of the SRF (p=0.00, p=0.00) was found to be statistically significant both at 2 and 3months respectively. The BCVA improvement at 2months in comparison to the baseline was found to be statistically significant (p=0.03). Recurrence was seen in 9/13 (69.23%) eyes at 6 months. No side effect of the drug was noted. In our placebo group an antioxidant containing lutein and zeaxanthin was given for 2 months, the mean duration of SRF was 9.38±9.86 months. 6 eyes out of 19 eyes (31.58%) showed a complete resolution of the SRF at 2 months. A statistically significant reduction of SRF height was seen at 1 and 2 months (p-value 0.005 and 0.043 respectively) and reduction of the horizontal SRF length at 2 and 3 months (p-value 0.035 and 0.025 respectively) compared to the baseline values. This may have resulted in a statistically significant visual acuity improvement at 2 months (p-value 0.028) as compared to the baseline, which showed further improved at 3 months (p-value 0.005). Out of the 6 eyes with complete resolution of SRF at 3 months, 2 eyes (33.33%) showed a recurrence at 6months. The choroidal thickness showed a statistically significant reduction (p-value 0.034 and 0.024) at 1 and 2months of follow up. Our study is the first prospective placebo controlled randomized study. On comparing, the various groups with each other we found that the rifampicin group showed a statistically significant reduction in choroidal thickness at all follow up visits. Spironolactone showed maximum reduction in the height and horizontal length of SRF as compared to the other two groups and this possibly resulted in a good visual recovery. It also showed the maximum number of the eyes with complete resolution of SRF as compared to the other two groups however the number of recurrences at 6months was the highest in this group. Placebo group showed the least number of recurrences of SRF at 6months follow up. This study has shown surprising results in the form of a significantly increased proportion of patients who recovered on treatment with spironolactone within two months. This difference was statistically and clinically significant. The limitation in our study is the sample size. Based on the absolute risk reduction, spironolactone has a NNT (Number Needed to Treat) of 5 only at the 6 months. The confounding factor here is the crossover that occurred after 2 months. This is also seen more dramatically when the recurrence rate is examined closely. The lowest recurrence with placebo and the highest with Spironolactone. The NNH (Number Needed to Harm) at 6 month time point is 2.2. The current data makes the use of spironolactone as a therapy in chronic CSCR untenable. Similarly, rifampicin too has a NNH close to 2.2 with resolution rates worse than placebo at alltime points. An intriguing possibility in our study is that the placebo chosen may have actually exerted a therapeutic effect. Although it is premature to state this with any degree of certainty, the data does suggest this. There is little evidence in literature to suggest the same. Shinojima et al., [20] in their randomized placebo controlled study treated 79 patients of chronic CSCR (>6 months) with 37 patients in the supplementation group (antioxidant with lutein) and 42 patients in the placebo group. SRF resolution was seen in 32.4% eyes in the supplementation group and 28.6% in the placebo group. There was no statistically significant difference between the 2 groups. The supplementation group showed significant improvement in BCVA. The SRF reduction rate was higher in the supplementation than in the placebo group at 6 months. Tachi N et al., [31] showed that the cessation of smoking and anti-oxidants help in resolving SRF. The major limitations of our study are small sample size, treatment with each drug for 2 months only, which may not be adequate to assess their role in the resolution of SRF in chronic CSCR and short follow up of 6 months after starting the drug. Further larger and controlled studies are indicated in order to validate these findings.
Conclusion
The patients of chronic CSCR have limited options of treatment and the resolution of SRF may not always translate into visual improvement due to an underlying unhealthy RPE at the macula. Spironolactone and placebo were comparable in the resolution of the SRF at 2 months. However, by the end of three months there was a statistically significant difference between spironolcatone and placebo, where spironolactone was found to be more efficacious in resolving SRF. The lesser efficacy of placebo was outweighed by the least number of recurrences at 6months thereby making placebo (antioxidants in our study) an attractive choice for the treatment of chronic CSCR. An interesting follow up study to do would be to compare antioxidant therapy with natural history in the prevention of recurrences. However, studies with a larger sample size maybe required in future to establish the role of these drugs in the management of chronic CSCR.
Acknowledgement
Institutional Ethical approval and consent for participation and publication was taken. The datasets used and/or analysed during the current study are available from the corresponding author. We have received no funding for this work from any organization. We have no financial interest to disclose. We have no conflict of interest with each other. The manuscript has been read and approved by all the authors. The requirements for authorship have been met, and each author believes that the manuscript represents honest work. All authors have contributed substantially.
The authors thank Dr Rupesh Agarwal, Consultant Ophthalmologist, Department of Ophthalmology and Tan Tock Seng Hospital, Singapore for reviewing and editing the manuscript. Written permission from person acknowledged has been obtained.
References
- Gass JD. Pathogenesis of disciform detachment of neuroepithelium: II. Idiopathic central serous choroidopathy. Am J Ophthalmol. 1967; 63: 587- 615.
- Marmor MF. New hypotheses on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1988; 226: 548-552.
- Kapoor KG, Sim J. Spironolactone for Secondary Central Serous Chorioretinopathy: A Challenge-Rechallenge Case. Case Rep Ophthalmol. 2017; 8: 370-374.
- Venkatesh R, Agarwal M, Kantha M. Efficacy of oral rifampicin in chronic central serous chorioretinopathy. Ther Adv Ophthalmol. 2018; 10: 2515841418807130.
- Shin JY, Woo SJ, Yu HG, Park KH. Comparison of efficacy and safety between half-fluence and full fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina. 2011; 31: 119-126.
- Ma J, Meng N, Xu X, Zhou F, Qu Y. System review and meta-analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmol. 2014; 92: e594-e601.
- Sivaprasad S, Elagonz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: evolution and clinical applications. Surv Ophthalmol. 2010; 55: 516-530.
- Manayath GJ, Ranjan R, Karandikar SS, Shah VS, Saravanan VR, Narendran V. Central serous chorioretinopathy: Current update on management. Oman J Ophthalmol. 2018; 11: 200-206.
- Bousquet E, Beydoun T, Rothschild PR, Bergin C, Zhao M, Batista R, et al. Spironolactone for Nonresolving Central Serous Chorioretinopathy. Retina. 2015; 35: 2505-2515.
- Sun X, Shuai Y, Fang W, Li J, Ge W, Yuan S, et al. Spironolactone versus observation in the treatment of acute central serous chorioretinopathy. Br J Ophthalmol. 2018; 102:1060-1065.
- Falavarjani KG, Amirsardari A, Habibi A, Eshaghi A, Bakhti S, Aghdam A. Visual and anatomical outcomes of spironolactone therapy in patients with chronic central serous chorioretinopathy. J Ophthalmic Vis Res. 2017; 12: 281-289.
- Kim JY, Chae JB, Kim J, Kim DY. Mineralocorticoid Receptor Antagonist Treatment for Steroid-Induced Central Serous Chorioretinopathy Patients with Continuous Systemic Steroid Treatment. J Ophthalmol. 2018; 2018: 4258763.
- Shulman S, Goldenberg D, Schwartz R, Wilner ZH, Barak A, Ehrlich N, et al. Oral Rifampin treatment for longstanding chronic central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol. 2016; 254: 15-22.
- Mahar PS, Memon N, Memon AS, Faheem MF. Role of Oral Rifampicin in Chronic Central Serous Chorioretinopathy. Pak J Ophthalmol. 2018; 34: 3-8.
- Meyerle CB, Freund KB, Bhatnagar P, Shah V, Yannuzzi LA. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina. 2007; 27: 943-946.
- Nielsen JS, Jampol LM. Oral mifepristone for chronic central serous chorioretinopathy. Retina. 2011; 31: 1928-1936.
- Singh RP, Sears JE, Bedi R, Schachat AP, Ehlers JP, Kaiser PK. Oral eplerenone for the management of chronic central serous chorioretinopathy. Int J Ophthalmol. 2015; 8: 310-314.
- Gruszka A. Potential involvement of mineralocorticoid receptor activation in the pathogenesis of central serous chorioretinopathy: case report. Eur Rev Med Pharmacol Sci. 2013; 17: 1369-1373.
- Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Cohen FB. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina. 2013; 33: 2096-2102.
- Shinojima A, Sawa M, Sekiryu T, Oshima Y, Mori R, Hara C, et.al. A multicenter randomized controlled study of anti-oxidant supplementation with lutein for chronic central serous chorioretnopathy. Ophthalmologica. 2017; 237: 159-166.
- Kim GA, Rim TH, Lee SC, Byeon SH, Koh HJ, Kim SS, et al. Clinical characteristics of responders to intravitreal bevacizumab in central serous chorioretinopathy patients. Eye (Lond). 2015; 29: 732-741.
- Ozdemir O, Erol MK. Morphologic changes and visual outcomes in resolved central serous chorioretinopathy treated with ranibizumab. Cutan Ocul Toxicol. 2014; 33: 122-126.
- Baran NV, Gurlu VP, Esgin H. Long-term macular function in eyes with central serous chorioretinopathy. Clin Experiment Ophthalmol. 2005; 33: 369-372.
- Garg SP, Dada T, Talwar D, Biswas NR. Endogenous cortisol profile in patients with central serous chorioretinopathy. Br J Ophthalmol. 1997; 81: 962-964.
- Nicholson B, Noble J, Forooghian F, Meyerle C. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol. 2013; 58: 103-126.
- Ravage Z, Packo K. Rifampicin for treatment of central serous chorioretinopathy. Invest Ophthalmol Vis Sci. 2011; 52: 2137.
- Sabouri MR, Kazemnezhad E. Evaluation of Therapeutic Effect of Rifampin for Acute Central Serous Chorioretinopathy. Iranian J Ophthalmol. 2014; 26: 102-107.
- Choudhury D, Sharma P, Dora J. Role of Rifampicin and Ketoconazole in Chronic Central Serous Chorioretinopathy: a Comparative Study. J Evid Based Med Healthc. 2016; 3: 3940-3944.
- Delyani JA. Mineralocorticoid receptor antagonists: the evolution of utility and pharmacology. Kidney Int. 2000; 57: 1408-1411.
- Gasparo MD, Joss U, Ramjoue HP, Whitebread SE, Haenni H, Schenkel L, et al. Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro. J Pharmacol Exp Ther. 1987; 240: 650-656.
- Tachi N, Ashikari M, Yasukawa T, Hirano Y, Ogura Y. The impact of stopsmoking and antioxidant supplements on patients with chronic central serous chorioretinopathy. Invest Ophthalmic Vis Sci. 2011; 52: 4511.