
Case Report
J Ophthalmol & Vis Sci. 2020; 5(2): 1042.
The Time Course of Retinal Ganglion Cells Layer Loss after Non-Arteritic Anterior Ischemic Optic Neuropathy (NAION)
Tran BK, Borruat FX*
Department of Ophthalmology, University of Lausanne, Switzerland
*Corresponding author: Francois-Xavier Borruat, Department of Ophthalmology, University of Lausanne, Hopital Ophtalmique Jules Gonin, Avenue de France 15, Lausanne 1004, Switzerland
Received: December 01, 2020; Accepted: December 18, 2020; Published: December 25, 2020
Introduction
Non-Arteritic Anterior Ischemic Optic Neuropathy (NAION) is a frequent acute optic neuropathy, affecting 2-10/100’000 patients over 50 years-old in USA [1]. It is characterized by loss of vision and permanent loss of Retinal Ganglion Cells (RGC). The classic presentation of NAION is a painless, acute or subacute unilateral visual loss with altitudinal visual field defect that most patients will notice upon awakening from sleep [2]. A small non-excavated optic disk (so-called disc-at-risk) is often found in the unaffected fellow eye and is thought to represent an anatomic predisposition to develop NAION. Acute ischemia of the retrolaminar portion of the optic nerve results from a deficient perfusion of posterior short ciliary arteries, but the exact mechanisms leading to the acute ischemic event are still unknown and are most likely multifactorial [3]. Whereas the primary cause of the ischemic event is not precisely known, the following sequences in NAION are nowadays accepted, based on both experimental data (rodent and non-human primate models of NAION) and clinical data. An initial ischemic axonal event is followed by an early inflammatory acellular response (cytokines, inflammatory proteins), then followed by a late inflammatory cellular response [4,5].
The visual prognosis of NAION is poor as only 30-40 % of patients may benefit from spontaneous but partial improvement of visual function. Furthermore, 15% of NAION will suffer from the progressive form of NAION with subsequent and additional loss of visual function occurring up to four weeks after the onset of NAION. Neuroprotection might help to prevent RGC apoptosis, hence potentially allowing to favorably influence the visual outcome of patients with NAION. The window of opportunity to treat an acute traumatic optic neuropathy has been estimated in animal models, but is not precisely determined in human NAION. Initially an eye affected by NAION exhibits swelling of the optic nerve head, sometimes sectorial, frequently accompanied by papillary flame hemorrhages. After a few weeks, the optic disk swelling subsides revealing sectorial atrophy corresponding to the defective visual field. Spectral-domain Optical Coherence Tomography (OCT) allows nowadays to perform in vivo assessments of either the peripapillary Retinal Nerve Fiber Layer (RNFL) or the macular RGC Layer (RGCL) thicknesses. In acute NAION, RNFL measurements are not helpful, as the RNFL swelling prevents to assess which axons are still functioning. Early RNFL measurements do not match the pattern of visual field defect. It is only at the stage of optic atrophy that both measurements will be in accordance [6]. On the other hand, the macula is usually not swollen in the acute stage of NAION and RGCL measurement is readily available to the clinician [7,8]. Visual field defects can be predicted earlier and more precisely from RGCL than from RNFL measurements. In animal models, the time-course of RGCL loss has been studied [9,10]. In humans only a few in vivo studies addressed the question of RGCL loss using OCT [11-13]. In these studies, the RGCL thickness was assessed initially, mostly at one month, then at 3-6 months later. Knowing the precise dynamics of RGC loss after NAION might be helpful for properly designing future therapeutic studies of NAION in humans. Depending on the SD-OCT machine, the segmentation program will allow either to isolate Retinal Ganglion Cell Layer (RGCL) or to measure altogether the retinal Ganglion Cell and Inner Plexiform Layers (GCIPL) For the present study we used Cirrus SD-OCT (Cirrus, Carl Zeiss Meditec AG, Jena, Germany) which algorithm calculates GCIPL thickness. We report the precise evolution of GCIPL and RNFL thickness in a patient with NAION serially investigated with OCT for 6 months, from Day 2 after NAION.
Case Presentation
A 47-year-old man complained of the acute onset of painless visual loss in his Left Eye (LE) for two days. He noticed visual loss in the morning, upon awakening. Seven years previously, he presented a similar event in his Right Eye (RE), diagnosed as NAION. His past medical history was unremarkable and he did not take any medications. He smoked 20 cigarettes/day for the past 30 years and denied the use of illegal substances or 5-phosphodiesterase inhibitors. Initial examination revealed normal visual acuity (20/20) and color vision (Ishihara 13/13) in both eyes (OU). Pupils were round and reactive without a relative afferent pupillary defect. Computerized visual field examination revealed almost symmetrical bilateral absolute inferior altitudinal defect (Figure 1, Top). Slit lamp examination was normal OU and intraocular pressure was 14mmHg RE and 15mmHg LE. Fundus examination revealed a sectorial superior atrophy of the right optic disk whereas the left optic disk was swollen and hyperemic (Figure 1, Bottom). Apart from leakage from the left optic disk, fluorescein angiography was normal OU, namely with normal retinal and choroidal circulation times OU. Assessment with OCT revealed a sectorial superior decrease of RNFL thickness RE and a diffuse increase in RNFL thickness LE. Evaluation of GCIPL revealed an altitudinal superior loss of RGCL RE matching perfectly the inferior visual field loss, whereas GCIPL thickness was completely normal LE (Figure 2).
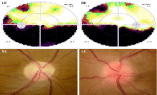
Figure 1: Initial examination was performed two days after the onset of visual
loss in the Left Eye (LE).
Top: Visual field examination revealed symmetrical absolute inferior altitudinal
visual loss in both eyes.
Bottom: Fundus examination showed the presence of sectorial superior optic
atrophy in the right eye (RE-left) and diffuse swelling of the left optic disk
(LE-right).
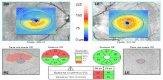
Figure 2: Initial OCT assessment (Cirrus, Carl Zeiss Meditec AG, Jena,
Germany).
Evaluation of RGCL revealed altitudinal superior loss in the RE but was
entirely normal in the LE.
For the next 6 months, visual function (visual acuity, color vision and visual field) was unchanged in both eyes. The patient kindly accepted to be serially examined with OCT during this follow-up time. Evolution of GCIPL thickness is plotted in (Figure 3). For the RE, there was no statistically significant change of either RNFL or GCIPL thicknesses, be it globally or sectorially. For the LE, RNFL thickness decreased gradually and exponentially over 12 weeks to reach a stable value similar both globally and sectorially to the RE. The dynamics of GCIPL thinning in the LE was characterized by 4 distinct phases. First, GCIPL thickness remained within normal limits although the measured thickness slowly decreased until Day 6 post NAION. Second, starting at Day 7, a rapid loss of GCIPL thickness occurred until Day 15. Third, a plateau of relative stability was observed from Day 16 until Day 44 post NAION. Fourth, a last phase of slow but significant decrease in GCIPL thickness was present until Month 4.5. At the end of the follow-up period, there were no interocular differences for either optic disk appearance, visual field defect, visual acuity, color vision, or OCT results (both GCIPL and RNFL loss).
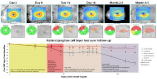
Figure 3: Evolution of RGCL thickness over time.
Top: Results of RGCL measurements between Day 2 and Month 4.5.
Bottom: Evolution of RGCL thickness on a logarithmic scale depicting the
four phases of RGCL loss observed in our patient. The top line refers to the
inferior sectors of RGCL thicknesses. The bottom line refers to the superior
sectors of RGCL thicknesses.
Discussion
Our patient presented sequential bilateral non-progressive NAION with ultimately no significant interocular differences, be it for either visual function (since initial examination) or loss of RNFL or GCIPL by OCT measurements at the final assessment. Despite immediate and permanent loss of visual field, the initial measurement of GCIPL at Day 2 remained within normal limit until Day 6. The delay to a measurable decrease in GCIPL thickness can be explained by the time for the retrograde axonal degeneration to reach the RGC [9]. Starting at Day 7, a phase of rapidly decreasing GCIPL thickness was observed until Day 15. This second phase corresponded most likely to RGC’s apoptosis. Indeed, a similar pattern of RGC loss has been observed experimentally. In rats, Villegas-Perez et al., were able to demonstrate a two-step manner of RGC loss after optic nerve injury: first a rapid loss of RGC within 2 weeks, followed by a slower and steady loss over one month [10]. Another group documented that optic nerve transection in rats resulted in an abrupt RGC apoptosis between Day 5 and 14 [14]. In humans, Kupersmith et al., reported permanent reduction of RGCL thickness within one month after the onset of NAION in 29 subjects, some patients developing the RGCL thinning within 2 to 3 weeks after presentation [11]. Similarly, Akbari et al., reported that thinning of the GCIPL was first evident at one month after the onset of visual symptoms [12]. On the other hand, De Dompablo et al., investigated prospectively a series of 16 patients with N-AION and reported RGC loss as early as 2 days after the onset of visual symptoms [13]. The last two steps of the curve of GCIPL loss in our patient are subjects to discussion. From Day 16 to Day 40 after presentation, the curve plateaued and one could argue that nearly all RGC’s were lost by Day 16. What is more disturbing is the last part of the curve, revealing a slow but steady decrease in GCIPL thickness from Day 40 to Month 4.5. This could represent either thinning of supportive, now unnecessary, tissue, or a second phase of RGC loss. Whereas common sense would favor loss of supportive tissue, experimental data in rodent models of optic neuropathy revealed that the number of RGC’s continued to decrease at a slow rate during a few months following optic nerve injury [15]. In our patient, the significance of this late decrease in GCIPL thickness is not known, as no clinical worsening was observed.
Up to now, there is no evidence-based medicine that any medical or surgical therapies are associated with a better visual outcome than observation alone [3]. Axonal loss probably cannot be prevented, as the ischemic event has already occurred by the time of diagnosis. However, neuroprotective therapy might be able to prevent the retinal ganglion cells to undergo apoptosis [16]. Preserving RGC’s might allow a possible reversal of visual loss. Knowledge of the dynamics of RGC loss after an acute onset of optic neuropathy is of utmost importance in order to intervene during the appropriate timeframe, hence increasing the chances of rescuing RGC’s. Both experimental data in rodents and the present in vivo study suggest that there is a therapeutic window to rescue RGC’s after optic nerve injury like NAION. The therapeutic window in humans might be as short as 6 days following ischemic injury.
References
- Arnold AC. Ischemic optic neuropathy. In: Miller NR, Newman NJ, Biousse V, Kerrison JB, eds. Walsh and Hoyt’s Clinical Neuro-Ophthalmology, 6th edition, Baltimore, MD: Lippincott-Williams & Wilkins. 2005; 1: 349-384.
- Hayreh SS, Podhajsky PA, Zimmerman B. Nonarteritic anterior ischemic optic neuropathy: time of onset of visual loss. Am J Ophthalmol. 1997; 124: 641-647.
- Kerr NM, Chew SS, Danesh-Meyer HV. Non-arteritic anterior ischaemic optic neuropathy: a review and update. J Clin Neurosci. 2009; 16: 994-1000.
- Zhang C, Guo Y, Miller NR, Bernstein SL. Optic nerve infarction and post Zhang C, Guo Y, Miller NR, Bernstein SL. Optic nerve infarction and postischemic inflammation in the Rodent Model of Anterior Ischemic Optic Neuropathy (rNAION). Brain Res. 2009; 1264: 67-75.
- Salgado C, Vilson F, Miller NR, Bernstein SL. Cellular inflammation in nonarteritic anterior ischemic optic neuropathy and its primate model. Arch Ophthalmol. 2011; 129: 1583-1591.
- Bellusci C, Savini G, Carbonelli M, Carelli V, Sadun AA, Barboni P. Retinal nerve fiber layer thickness in nonarteritic anterior ischemic optic neuropathy: OCT characterization of the acute and resolving phases. Graefes Arch Clin Exp Ophthalmol. 2008; 246: 641-647.
- Keller J, Oakley JD, Russakoff DB, Andorra-Ingles M, Villoslada P, Sanchez- Dalmau BF. Changes in macular layers in the early course of non-arteritic ischaemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2016; 254: 561-567.
- Aggarwal D, Tan O, Huang D, Sadun AA. Patterns of ganglion cell complex and nerve fiber layer loss in nonarteritic ischemic optic neuropathy by Fourierdomain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 4539-4545.
- Villegas-Perez MP, Vidal-Sanz M, Rasminsky M, Bray GM, Aguayo AJ. Rapid and protracted phases of retinal ganglion cell loss follow axotomy in the optic nerve of adult rats. J Neurobiol. 1993; 24: 23-36.
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. The J of Neurosci. 1994; 14: 4368-4374.
- Kupersmith MJ, Garvin MK, Wang JK, Durbin M, Kardon R. Retinal ganglion cell layer thinning within one month of presentation for non-arteritic anterior ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2016; 57: 3588-3593.
- Akbari M, Abdi P, Fard MA, Afzali M, Ameri A, Yazdani-Abyaneh A, et al. Retinal ganglion cell loss precedes retinal nerve fiber thinning in nonarteritic anterior ischemic optic neuropathy. J Neuroophthalmol. 2016; 36: 141-146.
- De Dompablo E, Garcia-Montesinos J, Munoz-Negreto FJ, Rebolda G. Ganglion cell analysis at acute episode of nonartitic anterior ischemic optic neuropathy to predict irreversible damage. A prospective study. Graefes Arch Clin Exp Ophthalmol. 2016; 254: 1793-1800.
- Kanamori A, Catrinescu MM, Belisle JM, Costantino S, Levin LA. Retrograde and Wallerian axonal degeneration occur simultaneously after retinal ganglion cell axotomy. Am J Pathol. 2012; 181: 62-73.
- Nadal-Nicolas FM, Sobrado-Calvo P, Jimenez-Lopez M, Vidal-Sanz M, Agudo-Barriuso M. Long-Term Effect of Optic Nerve Axotomy on the Retinal Ganglion Cell Layer. Invest Ophthalmol Vis Sci. 2015; 56: 6095-6112.
- Ahmed Z, Kalinski A, Berry M, Almasieh M, Ashush H, Slager N, et al. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis. 2011; 2: e173.