
Case Series
J Ophthalmol & Vis Sci. 2022; 7(1): 1062.
Postoperative Perfusion Findings in Highly Myopic Eyes: Case Series Report
Quiroz-Reyes MA¹*, Quiroz-Gonzalez EA², Morales-Navarro J², Nieto-Jordan A¹ and Graue- Wiechers F¹
1Retina Service, Institute of Ophthalmology Conde de Valenciana Foundation, National Autonomous University of Mexico, Mexico City, Mexico
2Department of Ophthalmology, Institute of Ophthalmology Conde de Valenciana Foundation, National Autonomous University of Mexico, Mexico City, Mexico
*Corresponding author: Miguel A. Quiroz-Reyes, Vitreoretinal and Macular Specialist and Surgeon, Institute of Ophthalmology, Conde de Valenciana Foundation, Chimalpopoca 14, Colonia Obrera, Mexico City, Mexico
Received: January 10, 2022; Accepted: February 08, 2022; Published: February 15, 2022
Abstract
Purpose: To examine the structural, functional, and perfusion immediate outcomes and long-term correlated follow-up perfusional outcomes in surgical patients at different stages of myopic traction maculopathy (MTM).
Methods: We performed a retrospective, comparative, interventional, one-surgeon, case-control study in 6 eyes of 6 patients enrolled between May 2018 and December 2021. Three groups of eyes were examined: one normal emmetropic eye (Control emmetropia), one healthy myopic eye (Control high myopia), and 4 operated and structurally fully resolved myopic eyes with different stages of MTM (Surgically treated group). Long-term follow-up postoperative functional and perfusion evaluations were performed with spectral domainoptical coherence tomography (SD-OCT) and OCT angiography.
Results: Six eyes of 6 patients were included in the study. In the surgical group the stage distribution was one eye at each stage of myopic traction maculopathy. The preoperative BCVA was 1.29±0.54 logMAR, and the postoperative BCVA was 0.60±0.52 logMAR (P<0.05), the axial length was 30.49±1.87 mm with a mean time to surgery of 19.3±16.21 months. The difference in perfusion indices across groups was statistically significant (p<0.005).
Conclusion: Better functional, structural and perfusion indices outcomes were observed when highly myopic eyes underwent surgery early. Due to the risk of developing irreversible vision loss when undergoing surgery in late stages of this condition, longitudinal fellow-eye structural and perfusional evaluation is advised to detect early stages of MTM and make a suitable surgical decision to optimize the visual outcomes.
Keywords: Choriocapillaris flow; Deep vascular plexus; High myopia; Foveal avascular zone; Myopic macular degeneration; Macular hole; Macular hole associated retinal detachment; Superficial vascular plexus; Vessel density
Introduction
High myopia is defined as myopia with at least -6.0 diopters of refractive error or axial length over 26.5mm. Characteristic features include Bruch’s membrane ruptures, retinal atrophy, and sclerotic thinning. Complicating factors include the presence of posterior staphyloma and schisis-like thickening of the macula [1]. When the Henle nerve fiber layer is elongated in high-myopic eyes with staphyloma, it is referred to as myopic traction maculopathy (MTM), or myopic foveoschisis (MF) [2,3]. Epidemiological studies have documented a startling increase in prevalence of myopia in the last 40 years. For example, East and Southeast Asia have seen prevalence estimates rise from a stable 10-30% before the 1960s to 80-90% as of 2013, with 10-20% meeting criteria for high myopia [4]. In the United States, the prevalence of myopia has statistically increased over a 30-year period to 42% by 2004, with 2% with a spherical equivalent </= -7.9 diopters. Even in countries where vision problems and blindness are not highly prevalent, like in Japan, pathological myopia is nonetheless the leading cause for monocular cases [5]. Sociodemographic factors contribute to the risk substantially, with female sex, urban dwelling, and higher education identified as risk factors for myopia [6]. Similar demographic differences can be found in experimental study samples [7,8].
Scleral alterations have been proposed as the driving force of posterior segment pathologies. Scleral thinning and localized ectasia from a reduction in the thickness of individual collagen fibers has been observed in myopic eyes [9,10]. MF is a relatively new term, which was first described by Panozzo and Mercanti [7] using optical coherence tomography (OCT) in terms of subtle macular changes, such as epiretinal membrane (ERM), vitreomacular traction (VMT), macular or foveal retinoschisis, retinal thickening, a partial-or fullthickness macular hole (MH) with or without retinal detachment. These scleral pathological alterations and subsequent increase in axial length may contribute to foveomacular retinoschisis that exacerbates pre-existing VMT. The VMTs are considered to be the sources of traction-related vitreoretinal interface abnormalities, such as ERMs, posterior cortex hyaloid remnants, and retinal vessel rigidity [2].
Parolini et al. [11,12] proposed a staging MTM evolution classification that describes four MTM retinal stages (labeled 1 to 4) and three foveal stages (labeled a to c). Retinal detachment is associated with foveal stages 3 and 4. Parolini et al.’s staging classification correlates with loss of visual acuity (best-corrected visual acuity, BCVA) [12]. Pathological significance of MTM is important to determine when to surgically treat these patients [13]. MF, as the earliest stage of MTM, is defined as a tractional elongation of the Henle nerve fiber layer and is present in approximately 9-34% of patients with pathological myopia [7,14,15]. The natural evolution of highly myopic eyes with macular or foveal retinoschisis and foveal retinal detachment (FRD) is the development of macular holes or MH [16-18].
In some patients, early-stage MTM remains stable for several years. However, in others it progresses to FRD and MH with subsequent visual impairment [2]. MF pathogenesis is not well understood, but tractional forces, when combined with staphyloma, appear to be the important contributors [19]. The proposed mechanism of action is that eyeball elongation causes increased axial traction, which causes stretching within the posterior retina. More rigid internal limiting membrane (ILM) and retinal vessels may contribute to damage. Furthermore, all these degenerative changes may occur within the context of posterior staphyloma [20,21].
Current treatment options for MTM are largely limited to pars plana vitrectomy (with modified fovea saved ILM or without ILM peeling and tamponade with silicone oil or gas) and macular buckling [13,22-25]. Vitrectomy, with the release of vitreoretinal traction, yields good results and visual recovery. Even so, some patients require multiple interventions to achieve anatomic and functional success. Early stage symptomatic MTM, such as MF, may progress to myopic FRD, a partial-thickness MH and/or a full-thickness MH without retinal detachment, and rhegmatogenous retinal detachment with a full-thickness MH (MHRD) within the natural course of the disease. [2,13].
The most successful management strategy is highly dependent on the accurate identification of the MTM stage, with early stages being the most tractable. However, experts disagree as to what stage represents the best time to perform macular surgery. Until now, there have been different surgical indications with variable anatomic and functional results [8,23,26,27]. Recently, qualitative and quantitative perfusional evaluation of vessel density (VD) and choriocapillaris flow patterns at the macular level has elucidated the evaluation and management of different macular pathologies [28-31]. The present study aims to contribute to the sparse published data on macular perfusion, VD, and choriocapillaris flow (perfusion indices) in different stages of successfully operated MTM compared to normal control subjects. We compared the indices of macular microcirculation in a normal emmetropic eye (Figure 1A to 1A3), normal myopic eye (Figure 1B to 1B2), and operated eyes in which the different stages of MTM resolved completely after macular surgery and minimized confounding variables with careful participant selection and matching.
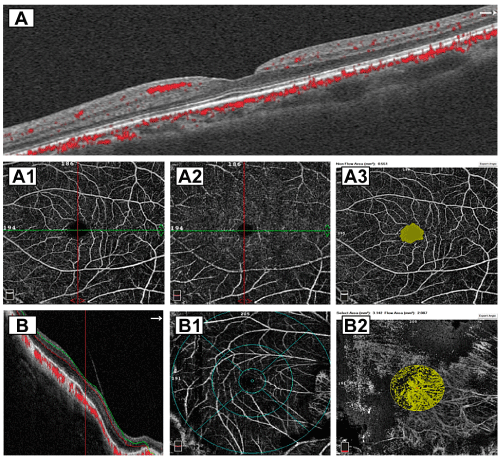
Figure 1: Normal control groups. A horizontal SD-OCT B scan with red dots
indicate intraretinal and choriocapillaris vascular flow in a normal emmetropic
eye. A1 and A2 corresponding normal SVP and DVP. A3 image depicts a
normal foveal a vascular zone (FAZ) of 0.551mm². B Horizontal B scan of
a highly myopic eye with automated red and green segmentation lines, the
posterior hyaloid membrane is still attached. B1 image depicts a quantitative
deep vascular plexus (DVP) slab with the ETDRS-like sectors grid overlay.
B2 image depicts a normal quantified area of choroidal flow with an irregular
but normal perfusion index at the selected subfoveal choriocapillaris.
The aim of this study was to comparatively evaluate the structural, functional, and perfusion immediate findings in normal control eyes with the long-term postoperative correlated follow-up perfusion outcomes in successfully resolved eyes at four different stages of symptomatic MTM.
Patients and Methods
Study design and patient selection
The manuscript reports on a retrospective analysis of medical records data obtained from the Retina Service at the Institute of Ophthalmology Conde de Valenciana Foundation in Mexico City between May 2018 and December 2021. Study protocol was approved by IRB of the facility; no approval was required for retrospective studies by this institution. Patients provided written informed consent for their data to be used for research purposes, consistent with standard institutional guidelines. Data included in the study are available from the Imagenology Laboratory at the retina department of the institution upon request.
Data for the surgery group was obtained from consecutively enrolled selected patients who underwent vitrectomy using different ILM surgical techniques for symptomatic MTM. Surgery was successful and uncomplicated in all cases.
The respective inclusion criteria were as follow: A normal emmetropic eye used as control eye, without previous disease history, BCVA of 20/20, normal Intraocular pressure and no structural abnormalities on the SD-OCT evaluation. A healthy highly myopic eye without clinical or tomographic evidence of MTM. The surgery group was composed of 4 patients, one eye at each stage of MTM: one eye at stage 1 exhibiting MF with macular thickening due to internal or external foveoschisis; Column-like formations are observed across the hypo-reflective space, as indicated by Benhamou et al. [32]. One eye at stage 2 with evidence of FRD as a main structural tomographic characteristic. One eye at stage 3 with evidence of a partial-or full-thickness MH but without tomographic evidence of macular detachment per se. and one eye at stage 4 with posterior pole rhegmatogenous retinal detachment associated with an MH, i.e., MHRD Eye.
The six study eyes were loosely matched on sociodemographic characteristics (i.e., age, age) as well as clinical and associated variables (e.g., study period or duration of follow-up). Eyes included in the study data did not receive intravitreal injections or laser photocoagulation treatments before or during the analyzed timeframe.
Patient evaluations were performed using the standardized evaluation protocol. Post operated eyes were followed up for more than six months and examined between four and twelve months until the last follow-up evaluation.
Ocular examinations
All patients underwent standard general ophthalmic and preoperative evaluations. These included BCVA; Amsler grid test; slit lamp biomicroscopic examination. We also performed a detailed fundus evaluation using a panfundoscopic contact lens and indirect ophthalmoscopy. Preoperative and postoperative Image acquisition was performed using a SD-OCT (RTVue-XR platform SD-OCT; Optovue, Inc., Fremont, CA, USA) and swept-source DRC (OCT Triton device, Topcon Medical Systems, Inc., Oakland, CA, USA). Cross-sectional images of the macular region were acquired in the horizontal plane through the foveal center. Axial lengths were evaluated using partial coherence laser interferometry (Zeiss IOL Master 700; Carl Zeiss Meditec AG, Oberkochen, Germany). Posterior staphyloma was confirmed using 2D B-scan ultrasonography (B-USG; US A and B, Quantel Medical, Du Bois Loli, Auvergne, France). Indirect ophthalmoscopy was also used to aid the diagnosis.
The BCVA in Snellen unit was converted to logMAR units using standard formulas.
We performed postoperative perfusion and quantitative VD and choroidal flow evaluations using an OCT angiography device (RTVue XR OCT Avanti with AngioVue Software; OptoVue, Inc., Fremont, CA, USA). It uses a specialized split-spectrum amplitudedecorrelation angiography software algorithm and acquires 70,000 A-scans/s to compose OCT angiography volumes consisting of 304 × 304 A-scans, achieving high axial resolution at depths of up to 5μm and minimizing motion artifacts. Each OCT angiography cube scan is comprised of 304 × 304 A-scans within a 3mm × 3mm square centered on the fovea, which yields 304 B-scans. Each B-scan output displayed an average of at least two individual scans. We used default retinal imaging settings and built-in projection artifact removal tools to perform image adjustment and segmentation. Segmentation for superficial vascular plexus (SVP), deep vascular plexus (DVP), outer retina layer, and choriocapillaris subfoveal plexus slabs (CSP) was performed in the AngioVue software. Imaging data were interpreted by an independent analyst. Scan quality was evaluated using the standard signal strength index (SSI) provided by the software: only scans with an SSI of > 46 were included.
The foveal avascular zone (FAZ) area in the SVP slab was evaluated by analyzing images saved as PNG files in the AngioVue system. Each FAZ area was automatically outlined following AngioAnalytics with angiometrics in the AngioVue software system to facilitate the measurements. Projection artifacts were automatically excluded with digital outlining of the FAZ in the SVP; the superficial FAZ area was quantitatively calculated. A built-in tool in the AngioVue system measured the VD [33,34], and a quantitative evaluation of the SVP and DVP at different subregion of the macula was then performed. VD and flow/perfusion indices were obtained using four en-face slabs of the retina. Perfusion indices were calculated as the proportion of the vessels area with blood flow over the cumulative area measured. Whole-macula VD and CSP area were calculated as density values within a 3mm×3mm square and a 1mm in diameter circle automatically selected by the algorithm in the foveal area, respectively.
Surgical procedures
In the surgical cases, a standard 25-gauge three-port pars plana vitrectomy was performed under local anesthesia plus general sedation. Eyes included in this study had an axial length of >26.5mm. Eyes had no evidence of A3 (patchy foveal-affected chorioretinal atrophy), A2 (diffuse macular chorioretinal atrophy), or myopic choroidal neovascularization, according to the ATN grading and classification system [35]. MTM staging was verified using spectral-domain OCT (SD-OCT) findings. Only eyes with posterior staphyloma determined by fundus examination and by B-scan ultrasonography were included. Surgeries were performed by the same surgeon (MAQR) with the adopted homogenized inclusion and exclusion criteria. All patients completed a postoperative follow-up period of at least six months to be included in the statistical analysis.
Surgical techniques performed in these patients are described in detail in a previous publication [36]. In brief, a set of modified ILM peeling techniques were used, including classical ILM removal in MF, foveal-sparing ILM in FRD and the inverted-flap ILM peeling technique in cases of MH and MHRD. All of these techniques were performed using a 25-gauge vitrectomy cut and suction instrument (Alcon Constellation Vision System); 25-gauge diamond-dusted membrane scraper; 25-gauge 0.44 forceps (Grieshaber Revolution DSP ILM forceps, Alcon Labs, Fort Worth, TX). ILM flap manipulation was facilitated by the use of a 25-gauge Finesse ILM flex loop microinstrument (Grieshaber, Alcon Labs).
Statistical analysis
Data were entered and processed in Microsoft Excel. For analysis, data were transferred to GraphPad Prism version 8.2.1 and SPSS for Windows version 28. Data were assessed for normality of distributions. Statistical tests were chosen based on data normality: i.e., One-Way Analysis of Variance (ANOVA) and the Kruskal Wallis test were chosen for parametric non-parametric analysis, respectively. Spearman correlation test was used to compare perfusion indices with the final visual outcome. Wilcoxon matched signed-rank test was used to compare preoperative and postoperative BCVA values in the surgical group (logMAR). For functional evaluations among stages and surgical variants, only the final postoperative BCVA was included in the statistical analysis. P-values of <0.05 were considered statistically significant.
Results
General outcome
In stage 1 (MF) eye, after surgery no evidence of MF, FRD or macular hole was found. The post-operative BCVA (0.48 logMAR) was statistically significantly better at follow-up, when compared with the pre-operative BCVA (0.70 logMAR) Table 1 and 2. In stage 2 (FRD) eye, the preoperative and post-operative BCVA were 1.00 logMAR and 0.30 logMAR, respectively, which differed significantly (P < 0.001). In stage 3 (MH) eye, the preoperative and post-operative BCVA was 1.60 logMAR, and 0.48 logMAR, respectively, which differed significantly (P < 0.001). In the stage 4 (MHRD) eye, the preoperative and post-operative BCVA was 2.00 logMAR, and 0.60 logMAR, respectively, which differed significantly (P < 0.001).
Study groups
Age
Preoperative BCVA (logMAR units)
Axial length (mm)
Post-operative follow-up months
Control emmetropic eye
54
0
20.53
-
Control myopic eye
62
0
29.45
-
Surgical group (n = 4)
Stage 1
55
0.7
31.54
15
Stage 2
52
1
30.38
17
Stage 3
63
1.6
30.1
15
Stage 4
59
2
29.94
30
p-value
0.012
<0.0001
<0.0001
>0.05
BCVA: Best-Corrected Visual Acuity.
Table 1: Patients’ demographic data and pre-operative clinical characteristics.
Post-operative BCVA values in surgically treated groups were significantly lower, compared to the emmetropic control groups (p < 0.0001) (Table 3). Stage two diseases ended up with better BCVA when compared to other three stages (P < 0.0001).
Structural, functional and perfusion analysis among eyes
A microstructural analysis of the SD-OCT findings was conducted based on the International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel recommendations [37]. Results of the perfusion, structural and functional outcomes across different stages of the diseases are summarized in the Table 2. Briefly, the superficial FAZ area, in the control emmetropic eye was significantly smaller when compared with all other eyes (p < 0.0001). The superficial foveal VD in the emmetropic eye differed only from Stage 3 and 4 of the disease (p < 0.0001). Deep foveal VD differed only between emmetropic eye and stage 3 disease eye (p = 0.0058). CSP flow area was significantly larger in the emmetropic group (p < 0.0001).
Study groups
Superficial FAZ area (mm2)
Superficial foveal VD (%)
Deep foveal VD (%)
Superficial parafoveal VD (%)
Deep parafoveal VD (%)
Superficial whole macula VD (%)
Deep whole macula VD (%)
Choriocapillaris flow area (mm2)
CSFT (μm)
Post-Op BCVA
Control emmetropic
0.33
27.17
31.39
58.76
59.17
56.93
58.5
2.51
246.4
0
Control high myopia
0.64 *
27.57
32.11
55.24*
54.98*
46.35 *
48.55*
2.25
264.9
0
Surgically treated (n=4)
Stage 1
0.74 *
28.53
31.13
49.66 *
51.53*
48.81*
50.13*
1.90 *
228.6
0.48*
Stage 2
0.77 *
28.95
31.45
49.71*
50.83*
50.26*
50.37*
1.85*
208.7*
0.30*
Stage 3
1.28*
21.27*
25.51*
37.14*
39.58*
40.29*
41.46*
1.35 *
194.70*
0.48*
Stage 4
1.66*
22.64*
23.71
27.91*
29.81*
31.41*
33.69*
1.35*
214.8*
0.60*
Comparison with control emmetropic (p values)
0.0001
0.0001
0.0058
0.0001
0.0001
0.0001
0.0001
0.0001
0.0001
0.0001
*Indicates where data differed significantly (p<0.05) from the control emmetropic eye. The BCVA in the control emmetropic and control high myopia eyes was used for comparison with post-Op BCVA in the surgical group. Abbreviations: FAZ: Foveal Avascular Zone; VD: Vessel Density; CSFT: Central Subfield Foveal Thickness.
Table 2: Quantitative evaluation of macular perfusion across study groups.
Decreased superficial foveal VD correlated with poor visual outcome in stage 4 disease eye (p = 0.014). Similarly, smaller CSP flow area was associated with poorer visual outcome (p = 0.048) in stage 3 disease eye. Also, CSFT tended to correlate negatively with the final BCVA as shown in Table 3. Preoperative and final BCVA values stratified by stage are compared in Table 4, which shows that final visual acuities were significantly better across all the study variables.
Study group
Superficial FAZ area, p value (r coefficient)
Superficial foveal VD, p value (r coefficient)
Deep foveal VD, p value (r coefficient)
Superficial parafoveal VD, p value (r coefficient)
Deep parafoveal VD, p value (r coefficient)
Superficial whole macula VD, p value (r coefficient)
Deep whole macula VD, p value (r coefficient)
Choriocapillaris flow area, p value (r coefficient)
CSFT, p value (r coefficient)
Stage 1
0.12
0.55
0.96
0.89
0.75
0.96
0.5
0.47
0.68
Stage 2
0.77
0.78
0.76
0.65
0.58
0.74
0.96
0.91
0.29
Stage 3
0.39
0.59
0.73
0.76
0.73
0.73
0.64
0.04*
0.71
Stage 4
0.5
0.01*
0.07
0.75
0.92
0.85
0.63
0.85
0.48
*Indicates the groups where a positive correlation to the final BCVA was observed (p<0.05). Abbreviations: FAZ: Foveal Avascular Zone; VD: Vessel Density; CSFT: Central Subfield Foveal Thickness; SD: Standard Deviation.
Table 3: The correlation of final visual outcome with the perfusion indices (vessel density and flow index). Data indicate p values and Spearman correlation co-efficient.
Stage of MTM
Preoperative (logMAR units)
Final (logMAR units)
P*
0.48
< 0.001
Stage 2 eye
1
0.3
< 0.001
Stage 3 eye
1.6
0.48
< 0.001
Stage 4 eye
2
0.6
< 0.001
Table 4: Comparison between preoperative and final BCVA by stage (logMAR).
Surgical cases
Surgical case 1: A 55-year-old symptomatic woman presented with complaints of metamorphopsia and progressive visual loss in her right eye. The visual loss occurred over the period of seven months. Preoperative right eye visual acuity was 20/100 (logMAR 0.70); refractive defect of -20+2.00 × 170; applanation ocular tension 11mmHg. The right eye had an axial length of 31.54mm with PS. Preoperative high-definition SD-OCT findings were consistent with schisis-like macular thickening, and absence of central subretinal macular fluid (Figure 2A). We performed macular surgery using the classical ILM peeling technique with BBG dye as an adjuvant, airfluid exchange, and a non-expandable 15% perfluoropropane gas. After a 15-month follow-up, the foveomacular region remained attached (Figure 2B-2E), with a final BCVA of 20/60 (logMAR 0.48) and extrafoveal nasal, residual ERM remnants were observed (Figure 2E). Several SD-OCT biomarkers were noted, such as an irregular foveal contour and internal and external neuroretina lines without total restoration of the central subfoveal ellipsoid, such as at the IS/OS line and the external limiting membrane. The long-term postoperative perfusion evaluation was abnormal with lower-thannormal perfusion indices on the SVP slab (Figure 2F) and DVP slab (Figure 2G). The choriocapillaris flow area was considered in range with 2.087mm² (Figure 2H) with an enlarged and irregular FAZ of 0.551mm² (Figure 2I).
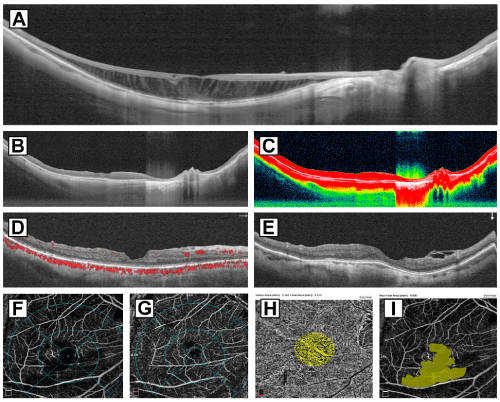
Figure 2: Surgical case 1: A 55-year-old symptomatic woman complained
of metamorphopsia and progressive visual loss in her right eye over seven
months. Her preoperative right eye visual acuity was 20/100 (logMAR 0.70)
with a refractive defect of -20.00+2.00 × 170 and applanation ocular tension
of 11mmHg. The right eye had an axial length of 31.54mm with PS. A image
shows a high definition 12mm horizontal b scan with schisis-like macular
thickening and tractional elongation of the Henle nerve fiber layer. The patient
underwent macular surgery with classical ILM peeling technique using a BBG
dye as an adjuvant to identify precisely the ILM and complete a modified
foveal sparing ILM technique, and a non-expandable 15% perfluoropropane
gas mixture. B and C images depict that the foveomacular region remained
attached after 15 months with a final BCVA of 20/60 (logMAR 0.48). D
image shows a horizontal b scan with an irregular foveal profile, well defined
ellipsoid zone and external limiting membrane line. E imagen shows the
corresponding vertical b scan with extrafoveal nasal, residual ERM remnants
was seen. F and G images indicate lower than normal perfusion indices on
the SVP and DVP. H image shows a choriocapillaris flow area of 2.113mm²
considered between range. I image depicts an enlarged and irregular FAZ of
4.036mm² above the normal range.
Surgical case 2: A 52-year-old symptomatic woman presented with complaints of aggravating metamorphopsia; these were accompanied by a progressive drop in central vision, and high myopia; PS observed in both eyes. The right eye with an axial length of 30.38mm underwent macular surgery due to a 9-month history of symptomatic myopic FRD (Figure 3A). The preoperative BCVA was measured at 20/200 (logMAR 1.00). We performed a three-port 25-G pars plana vitrectomy and non-foveal ILM peeling with the foveal sparing technique on this eye. Fluid-air gas exchange was performed with 15% C3F8 tamponade. After a 17-month longitudinal follow-up, the operated eye showed a postoperative BCVA of 20/40 (logMAR 0.30). The postoperative perfusion indices at the SVP slab (Figure 3B) and DVP slab (Figure 3C) were considered below mean with no evidence of recurrent FRD, or progression to MH in the SS and SDOCT (Figure 3D), the en-face superficial aspect of the retina show a heterogeneous thin RPE, the eye showed a normal choriocapillaris flow of 2.113mm² (Figure 3E) and an irregular and enlarge FAZ of 4.036mm² (Figure 3F).
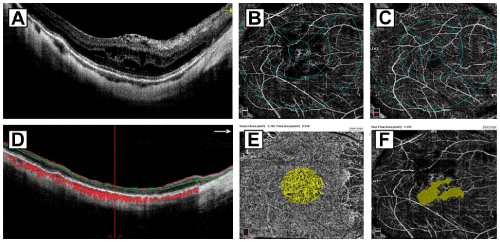
Figure 3: Surgical case 2: A 52-year-old symptomatic woman presented with
aggravating metamorphopsia, a progressive drop in central vision, and high
myopia. The right eye with an axial length of 30.38mm underwent surgery
because of a 9-month history of symptomatic myopic FRD (image A). The
preoperative BCVA was 20/200 (logMAR 01.00); this eye that underwent a
three-port 25-G pars plana vitrectomy and non-foveal ILM peeling with the
foveal sparing technique, fluid-air gas exchange was performed with 15%
C3F8 tamponade. B and C images depict the superficial vascular plexus and
deep vascular plexus with lower than mean perfusion indices, the operated
eye showed a postoperative BCVA of 20/40 (logMAR 0.30). D image depicts
automated red and blue segmentation lines horizontal B scan with diffuse
thinning of the superficial retinal layers and with no evidence of recurrent
FRD, or progression to MH, red dots represent choroidal flow. E image shows
a normal choriocapillaris flow of 2.270mm². F image depicts an irregular and
enlarge FAZ of 1.443mm².
Surgical case 3: A 63-year-old man presented with five months of disabling metamorphopsia, high myopia, and moderate PS. The right phakic eye underwent a 25-G three-port pars plana vitrectomy and macular surgery due to a full-thickness MH (Figure 4A). A modified BBG dye-assisted ILM peeling technique (inverted flap) and a 15% C3F8 long-acting non-expandable gas tamponade were used in this case. The preoperative BCVA was 20/800 (logMAR 1.60) and an axial length of 30.10mm. After a 15-month follow-up, the final postoperative BCVA was 20/60 (logMAR 0.48). The eye showed a normal macular profile and SD-OCT biomarkers such as inner and outer retina layers, subfoveal IS/OS, ELM, and a well-preserved RPE layer (Figure 4B and 4C), the perfusion evaluation was abnormal in both plexuses (Figure 4D and 4E) with perfusion indices lower than normal, the FAZ was irregular and enlarged with a size of 2.848mm2 (Figure 4F), the choriocapillaris flow was notably deficient with 1.964mm² in flow area (Figure 4G).

Figure 4: Surgical case 3: A 63-year-old man with five months of disabling
metamorphopsia, high myopia, and subsequently with severe drop vision. A
image depicts a high definition enhanced gray scale 12mm horizontal b scan
of a preoperative SD-OCT of the affected eye having a severe full-thickness
macular hole with persistent traction, epiretinal membrane proliferation and
inner and outer retinal layer schisis-like thickening with severe tractional
elongation of the Henle nerve fiber layer. This eye underwent vitreous and
macular surgery consisting of a modified BBG dye-assisted ILM peeling
technique (inverted flap) and a 15% C3F8 long-acting non-expandable gas
tamponade. The preoperative BCVA was 20/800 (logMAR 1.60) and an axial
length of 30.10 mm. After a 15-month follow-up, the final postoperative BCVA
was 20/60 (logMAR 0.48). B and C image show long-term postoperative B
scans with abnormal macular profile and presence of identifiable SD-OCT
biomarkers such as inner and outer retina SD-OCT layers, subfoveal IS/
OS, ELM line, and a well-preserved RPE layer. D and E images depict
comparison between superficial and deep vascular plexuses, both below
normal range. F image shows an abnormal FAZ of 2.848mm². G image
depicts the choriocapillaris flow of 1.964 mm² considered lower than normal
in range.
Surgical case 4: A 59-year-old woman with two months of slowly progressive decrease vision with metamorphopsia, high myopia, and severe PS. The right phakic eye underwent a 25-G three-port pars plana vitrectomy and macular surgery for macular hole related three quadrants rhegmatogenous retinal detachment (Figure 5A). The preoperative SS-OCT confirmed a MHRD (Figure 5B). This eye underwent vitreous and macular surgery consisting of BBG dye-assisted ILM peeling technique (inverted flap ILM peeling technique), after reapplication of the detached retina and a 15% C3F8 long-acting non-expandable gas tamponade. The preoperative BCVA was 20/2000 or counting fingers @ 2 feet (logMAR 2.00), with PS and an axial length of 29.94 mm. After a 30-month follow-up, the final postoperative BCVA was 20/80 (logMAR 0.60) and showed a SD-OCT pattern consistent with an abnormal macular profile and presence of biomarkers such as inner and outer retina SD-OCT layers, subfoveal IS/OS, ELM line and a well-preserved RPE layer (Figure 5C and 5D), the en-face aspect depicted multiple deep defects at the level of the RPE with a very abnormal perfusion evaluation on the SVP slab (Figure 5E) with better perfusion evaluation on the DVP slab (Figure 5F), the FAZ looked irregular and enlarge with 3.561mm2 in area (Figure 5G), the choriocapillaris flow was very deficient with 1.366mm² of flow from a selected area of 3.142mm² (Figure 5H).
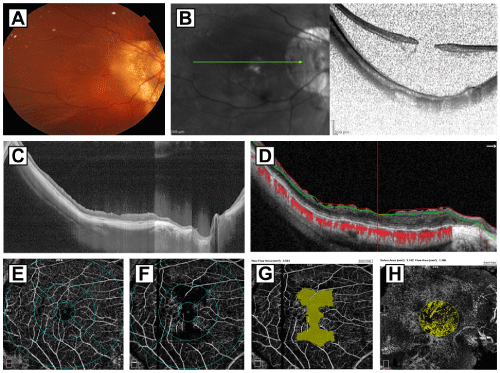
Figure 5: Surgical case 4: A depicts a color fundus photo of a 59-yearold
woman with two months of slowly progressive decrease vision with
metamorphopsia, high myopia, and severe an extensive three quadrants
rhegmatogenous retinal detachment associated to macular hole. B image
of a spectralis B scan examination consistent with retinal detachment and
confirmed presence of full-thickness macular hole. This patient underwent
25-G three-port pars plana vitrectomy and macular surgery consisting of BBG
dye-assisted ILM peeling technique (inverted flap ILM peeling technique),
after reapplication of the detached retina and a 15% C3F8 long-acting
non-expandable gas tamponade. The preoperative BCVA was 20/2000
(logMAR 2.00), with PS and an axial length of 29.94mm. C and D 30-months
postoperative images depict high definition 12mm horizontal scans in gray
scale with retina totally reattached and the macular hole closed, abnormal
macular profile, presence of SD-OCT biomarkers such as inner and outer
retina SD-OCT layers, disrupted subfoveal IS/OS (ellipsoid zone), irregular
external limiting membrane line and a well-preserved RPE layer. D image
depict automated red and green segmentation lines with dissociated optic
nerve fibers defects and irregular thinning of the inner layer of the retina.
The red dots represent choroidal flow. After a 14-month follow-up, the final
postoperative BCVA was 20/80 (logMAR 0.60). E and F images depict
superficial and deep vascular plexuses considered lower than normal at
the deferent macular subfields. G image shows an irregular and enlarged
superficial foveal avascular zone of 3.561mm². H image depicts deficient
choriocapillaris flow index of 1.366mm² considered under the normal range
value.
Discussion
Despite growing body of evidence documenting results from retrospective observational studies and a handful of interventional studies on MTM, our understanding of this condition, its clinical course, and its optimal management is still limited. Surgical treatments for MTM and its complications remain limited, and our understanding of these conditions is hindered by the lack of prospective trials and a small size of reported case series published to date. Although vitreomacular traction is the likely primary mechanism for the development of MTM, little has been written in relation to potential perfusion mechanisms behind MTM. Thus, vitrectomy and its variant techniques with or without ILM removal and the use of different types of tamponades are the most appropriate approaches [15]. For this reason, the present study aimed to report a long-term quantitative evaluation of VD and flow index at the macular level at different stages of successfully surgically operated MTM compared with normal controls. Our OCT angiography findings in perfusion indices provided perfusion data from surgically resolved eyes at different stages of MTM.
In this study, we relied on different saved ILM and removal techniques. Similarly to Al-Sheikh et al. [38], we observed a generally decreased choroidal perfusion in myopic eyes.
Müller cell integrity has been implicated in traction maculopathies. MTM is pathology of the Müller cell cones. It implicates inner traction from ILM and outer stretch forces from the PS, e.g., as described by Wang et al.[18] Structural changes in Müller cells are found in eyes with retinoschisis [39]: as retinoschisis cavities enlarge, mechanical stretch forces damage Müller cells and alter their function.[40] Our findings are consistent with Müller cell dysfunction account of visual impairment in eyes with retinoschisis-type MTM, as Wang et al. stated [41].
Shimada et al. [17] performed a prospective and observational study of 8 eyes with macular retinoschisis over an average follow-up of 44 months and detected a MH in two eyes. In one of them, retinal detachment was associated with macular hole. While both eyes started with only foveomacular retinoschisis, both eyes developed partial detachment of posterior hyaloid with persistent traction at the foveal level, and the rest of the patients in this study showed only progressive thickening of macula with data indicating foveomacular retinoschisis. Although its natural evolution to macular hole formation has been quite well described recently, its multiple pathogenic mechanisms have not been satisfactorily described.
Most patients with stage I disease or MF presenting to a macula specialist may be relatively asymptomatic, and the condition may persist at this stage for many years before affecting vision significantly [30,42]. Therefore, we agree with Takano and Kishi [21], Shimada et al. [17] and Ichibe et al. [42] who proposed that MF is the earliest stage or direct precursor lesion of FRD, or stage I disease in our report. Uchida et al. [20] showed that at this stage, 80% of the cases resolved with only vitrectomy and gas tamponade, and in 20% of cases, it was necessary to add ILM peeling techniques. We report the total resolution of foveoschisis in this eye, although a modified ILM peeling technique was implemented with good anatomic and functional outcomes. Hayashi et al. [43] observed the gradual progression of foveomacular retinoschisis to FRD in 41% of patients over a twelve-month follow-up period. In patients with documented MTM, the rate of progression to FRD from an earlier stage has been reported as 34.5%, and that of progression to partial-thickness MH has been reported as 20.7% [14]. Focal irregularities and the thickness of the external retina are frequent initial findings. These are usually followed by the formation of an outer lamellar defect associated with small focal retinal detachment. The column-like structures exert traction, leading to the enlargement of the lamellar defect. This, in turn, elevates the upper edge of the retina and causes the enlargement of the FRD [43,44] Baba et al. [45] reported a 9% incidence of FRD in patients with PS.
In a large case series [7], Panozzo and Mercanti found that the surgical resolution of vitreoretinal traction during the early stage of myopic FRD would allow re-flattening of the macular center. This would in turn prevent the development of partial- or full-thickness MH. The surgical preservation of foveal ILM may prevent FRD progression to advanced stages [25,46,47]. However, their series did not involve perfusion and qualitative evaluations using fluorescein angiography and quantitative VD evaluation using the AngioVue with OCT angiography as the study by Wang et al. [18] as well as our study did. Therefore, only the mechanical tractional alteration was largely proposed while not accounting for the possible role of macular and choroidal perfusion abnormalities with lower-than-normal quantified perfusion values. Choroidal perfusion support (increased VD in the choroid layer) can sustain MF with a low rate of MTM progression and prevent FRD formation [48]. Al-Sheikh et al. [38] suggested that decreases in choroidal perfusion and perturbations in VD in highly myopic eyes with a reduced whole-macula VD may be attributable to the mechanical stretch forces arising from the elongation of the eyeball.
As can be seen from our results as well as from the growing literature, quantitative evaluation of perfusion indices for assessment of macular microcirculation is of key clinical importance since mild perturbations in microcirculation can have negative consequences for vision quality due to induced pathological changes [49]. Smallvessel changes with accompanying lower VD values have been observed and document in multiple retinal vascular diseases to date, including diabetic retinopathy, macular telangiectasia, and radiation retinopathy [50-52].
In this study, owing to the reliability and the reproducibility of OCT angiography quantitative perfusion indices, we were able to detect reliable effects. Our study found that BCVA values were positively correlated with the CSP flow area. At the same time, BCVA values were correlated negatively (albeit at the level of clinical observation) with CSFT. Convergently, analysis performed using the logMAR units showed a negative correlation with the CSP flow area and a positive correlation with CSFT. However, these effects were not significant mainly due to the limited numbers of surgical patients. In the surgical group, we examined the correlations between BCVA and other OCT biomarkers, but none emerged as statistically significant effects.
We compared normal-range vessel changes in three groups of eyes: normal emmetropic; healthy highly myopic and eyes at different stages of surgically resolved MTM spectrum. We established significant differences in macular perfusion indices between the control eyes and between the different stages in MTM study group. We found highly significant effects when analyzing the microcirculation of the macula using quantitative VD indices. The difference between healthy eyes, and surgically resolved at the different stages of MTM was highly significant (p < 0.001). These findings suggest a possible relationship between tractional mechanisms and perfusion mechanisms without necessarily postulating directionality. We note that these effects should be replicated in future studies.
Peng et al. [53] speculated that upregulation of local cytokine levels drives the resolution of myopic FRD after fovea sparing ILM removal. It is therefore possible that he vascular microenvironment together with alterations in microcirculation permeability play an important role in MTM. Our study provides insights into the different stages of MTM, highlighting the role of microcirculation as a fundamental player in tractional pathogenesis. Specifically, this modified technique is recommended in both refractory and primary FRD cases accordingly with the author’s surgical and clinical experience. Modified ILM techniques result in foveal reattachment and are associated with significant improvements in visual acuity [23,26,27,30].
Several studies have directly examined the correlations between choroidal perfusion and VD measurements. Care should be taken to not reduce the choroidal perfusion in eyes with advanced MTM due to surgical changes (e.g., by compression of the choroid secondary to macular buckling surgery) [18]. FAZ distortion with enlargement of the yuxtafoveal capillary net contribute to decreased VD perifoveal perfusion indices in the different stages of MTM [36].
In our study and the study by Wang et al. [48] there was noted a positive correlation between BCVA and macular perfusion indices in the SVP slab and the 1mm selected foveal area of the choroid capillary layer across the different stages of MTM in the long-term postoperative perfusion evaluation as compared with controls at the end of follow-up. These findings suggest that vascular density flow abnormalities as reflected in a low value of choroidal thickness may directly lead of declines in visual acuity.
Development of a full-thickness MH may be spontaneous (i.e., as a part of the natural course of the disease) or secondary to ILM removal (i.e., in the case of classical macular surgery) [10,21,23,32]. Gaucher et al. [22] stated that the pathogenesis of MH formation might be different from that of idiopathic MH formation. Myopic eyes with total posterior vitreous detachment are subject to substantial traction exerted by residual cortical remnants on the macula. Thus, MH formation is preceded by FRD, where the foveola becomes extremely thin and the tangential traction exerted by a tense ILM / posterior vitreous remnants are associated with the occurrence of a MH.
Rhegmatogenous retinal detachment associated with MH formation presents a therapeutic challenge. Several surgical approaches are routinely used, including vitrectomy, retinal membrane peeling, and ILM peeling with modified peeling techniques and various tamponades. Several surgical approaches have also been described, including gas tamponade, silicone oil tamponade, additional laser treatment of the hole margin, and episcleral buckling of the macula area [54-56].
The reported success rate of vitrectomy with long-acting gas for eyes with MHRD varies between 45 and 68%, but higher success rates of up to 79% and 89% have been described with silicone oil as tamponade.[57-60] As mentioned by other authors [58], lack of chorioretinal tissue loss and RPE atrophy contribute to both a success in reapplication of the retina and better visual recovery in addition to considering the severe alterations in the perfusion indices found in our case of stage 4 MTM. Kadonosono et al. [61] revealed that myofibroblasts on the ILM surface contract around these macular holes, resulting in the ILM or ILM remnants playing a role in hole reopening, delayed hole closure or even subsequent retinal detachment; thus, the type of tamponade plays a secondary role in the incidence of macular hole closure and in depurative macular surgery, with the ILM peeling technique as the main factor of hole closure. It has also been reported that removing the ILM and overlaying ERM may increase the flexibility of the detached retina, in this way, contributing to MH sealing, even in the presence of posterior staphyloma [62-64].
As noted above, this study has several strengths, such as relying on data from patients with long-term follow-up evaluation and our ability to detect this condition early.
The study has a few limitations. First and foremost, we relied on a limited number of eyes. We note that this limitation was primarily due to the strict inclusion criteria applied to limit our study to successfully surgically corrected eyes at each one of the four different stages of MTM. Although this study lacks longitudinal analysis, its advantage is in combining SD-OCT and OCT angiography long-term findings, performing comparative quantitative evaluation of macular perfusional changes. We hope to contribute to the literature by addressing an important gap associated with the sparsity of published material on perfused macular assessment at the four main different stages of operated eyes with MTM in highly myopic eyes.
In summary, according to the perfusion, structural and functional outcomes in this case report, there was significant visual improvement when the eye was surgically treated in early stages of this condition, against a poor postoperative visual improvement (p<0.05) when the eyes underwent surgery for advanced stages of MTM. Likewise, perfusion indices were significantly lower when the eyes were operated on at advanced stages (p<0.005). This risk is higher when abnormalities in the perfusion indices have been detected in the postoperative period suggesting poor recovery prognosis. Thus, careful prospective and longitudinal eye evaluations are advised to detect early stages of this condition. This early identification is pivotal for making early and evidence-guided surgical choices while considering different macular surgical techniques to optimize the visual outcomes.
Conclusion
Further studies are needed to characterize the role of perfusion macular mechanisms in the pathogenesis of MTM. Of particular value will be prospective, randomized multi-center studies evaluating perfusion and histopathological findings. These studies can further elucidate role of microvascular perfusion mechanisms in maintaining the Müller cell integrity in traction maculopathies.
Declarations
Availability of data and materials: Photos, composite figures, datasets, and laboratory studies supporting the findings of this study may be released upon written application to the Photographic Laboratory and Clinical Archives Department at the Institute of Ophthalmology. Conde de Valenciana Foundation (non-profit organization), Chimalpopoca 14, Colonia Obrera, Mexico City, Mexico 06800, and the corresponding author upon request.
Authors’ contributions: Miguel Angel Quiroz-Reyes contributed to the study conceptualization and design of the study. The surgeries were performed by Miguel Angel Quiroz-Reyes. Material preparation, data collection, and analysis were performed by Miguel Angel Quiroz-Reyes, Erick Andres Quiroz-Gonzalez, Jorge Morales- Navarro, Alejandra Nieto-Jordan and Federico Graue-Wiechers. The first and main draft of the manuscript was written by Miguel Angel Quiroz-Reyes, and all authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript for publication.
Co-Authors Affiliation: All co-authors are affiliated to the Faculty of Medicine in the Postgraduate Division at NATIONAL AUTONOMOUS UNIVERSITY OF MEXICO. Mexico City, Mexico
Ethics approval: All procedures in this study involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the Declaration of Helsinki (1964) and its later amendments or comparable ethical standards. This retrospective study received full ethical approval from the research ethics boards and was approved by the institutional review committee and the teaching department of the institution enrolled (no reference number is provided for retrospective studies by this institution). Written informed consent was obtained from all patients in accordance with the institutional guidelines.
Consent to participate: Informed consent was obtained from all participants included in the study.
Consent for publication: The authors affirm that the participants provided informed consent for the publication of all images in Figure 1 to 5, as well as the images in the online resources, if any.
Acknowledgements: We express our deep appreciation to the technical staff of the participating institution: Retina Service at the Institute of Ophthalmology, Conde de Valenciana Foundation, Mexico City. Mexico, which is affiliated to The Postgraduate Division Studies at National Autonomous University of Mexico.
References
- Vitale S, RD Sperduto and FL Ferris. Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Archives of ophthalmology. 2009; 127; 12: 1632-1639.
- Ikuno Y and Y Tano. Early macular holes with retinoschisis in highly myopic eyes. American journal of ophthalmology. 2003; 136: 741-744.
- Sayanagi K, et al. Retinal vascular microfolds in highly myopic eyes. American journal of ophthalmology. 2005; 139: 658-663.
- Morgan IG, et al. The epidemics of myopia: aetiology and prevention. Progress in retinal and eye research. 2018; 62: 134-149.
- Xu L, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology. 2006; 113: 1134. e1-1134. e11.
- Xiang Z-Y & Zou H-D. Recent epidemiology study data of myopia. Journal of Ophthalmology. 2020; 7: 1-12.
- Panozzo G and A Mercanti. Optical coherence tomography findings in myopic traction maculopathy. Archives of ophthalmology. 2004; 122: 1455- 1460.
- Panozzo G and A Mercanti. Vitrectomy for myopic traction maculopathy. Archives of Ophthalmology. 2007; 125: 767-772.
- Curtin BJ. The myopias. Basic science and clinical management. 1985.
- Rada JAS, S Shelton and TT Norton. The sclera and myopia. Experimental eye research. 2006; 82: 185-200.
- Parolini B, et al. Myopic traction maculopathy: a new perspective on classification and management. The Asia-Pacific Journal of Ophthalmology. 2021; 10: 49-59.
- Parolini B, et al. The new myopic traction maculopathy staging system. European Journal of Ophthalmology. 2020: 1120672120930590.
- Quiroz-Reyes MA, et al. Long-Term Postoperative Structural and Functional Evaluation in Myopic Foveoretinal Detachment. Int J Ophthalmol Clin Res. 2021; 8: 132.
- Ikuno Y, F Gomi and Y Tano. Potent retinal arteriolar traction as a possible cause of myopic foveoschisis. American journal of ophthalmology. 2005; 139: 462-467.
- Todorich B, et al. Macular retinoschisis associated with pathologic myopia. 2013; LWW: 678-683.
- Sun C, et al. Natural evolution from macular retinoschisis to full-thickness macular hole in highly myopic eyes. Eye. 2010; 24: 1787-1791.
- Shimada N, et al. Natural course of myopic traction maculopathy and factors associated with progression or resolution. American journal of ophthalmology. 2013; 156: 948-957.e1.
- Wang S-W, et al. Biomarkers in the pathogenesis of epiretinal membrane and myopic traction maculopathy: Effects of internal limiting membrane incompliance and posterior staphyloma. Photodiagnosis and Photodynamic Therapy. 2021; 33: 102208.
- Oie Y, et al. Relation of posterior staphyloma in highly myopic eyes with macular hole and retinal detachment. Japanese journal of ophthalmology. 2005; 49: 530-532.
- Uchida A, et al. Vitrectomy for myopic foveoschisis with internal limiting membrane peeling and no gas tamponade. Retina. 2014; 34: 455-460.
- Takano M and S Kishi. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. American journal of ophthalmology. 1999; 128: 472-476.
- Gaucher D, et al. Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. American journal of ophthalmology. 2007; 143: 455-462. e1.
- Kobayashi H and S Kishi. Vitreous surgery for highly myopic eyes with foveal detachment and retinoschisis. Ophthalmology. 2003; 110: 1702-1707.
- Alkabes M and C Mateo. Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2018; 256: 863-877.
- Shimada N, et al. Fovea-sparing internal limiting membrane peeling for myopic traction maculopathy. American journal of ophthalmology. 2012; 154: 693-701.
- Kwok A, T Lai and W Yip. Vitrectomy and gas tamponade without internal limiting membrane peeling for myopic foveoschisis. British Journal of Ophthalmology. 2005; 89: 1180-1183.
- Sayanagi K, Y Ikuno and Y Tano. Reoperation for persistent myopic foveoschisis after primary vitrectomy. American journal of ophthalmology. 2006; 141: 414-417.
- Ho AC, et al. Intraretinal leakage of indocyanine green dye. Ophthalmology. 1994; 101: 534-541.
- Hwang TS, et al. Visualization of 3 distinct retinal plexuses by projectionresolved optical coherence tomography angiography in diabetic retinopathy. JAMA ophthalmology. 2016; 134: 1411-1419.
- Quiroz-Reyes MA, et al. Structural and Perfusional Study of Successfully Repaired Diabetic Tractional Retinal Detachment Involving the Macula. International Journal of Ophthalmology & Visual Science. 2021; 6: 187.
- Quiroz-Reyes MA, et al. Outcomes for Successfully Repaired Macula-Off Diabetic Tractional Retinal Detachment. Int J Ophthalmol Clin Res. 2021; 8: 131.
- Benhamou N, et al. Macular retinoschisis in highly myopic eyes. American journal of ophthalmology. 2002; 133: 794-800.
- Klufas MA, et al. Optical coherence tomography angiography reveals choriocapillaris flow reduction in placoid chorioretinitis. Ophthalmology Retina. 2017; 1: 77-91.
- Kuehlewein L, et al. Optical coherence tomography angiography of type 1 neovascularization in age-related macular degeneration. American journal of ophthalmology. 2015; 160: 739-748. e2.
- Ruiz-Medrano J, et al. Validation of the recently developed atn classification and grading system for myopic maculopathy. Retina (Philadelphia, Pa.). 2020; 40: 2113.
- Quiroz-Reyes MA, et al. Structural and Perfusional Findings in Surgically Resolved Myopic Foveoretinal Detachment Eyes and those in Early Stages of Myopic Traction Maculopathy. J Clin Exp Ophthalmologica. 2021; 12: 897.
- Staurenghi G, et al. International Nomenclature for Optical Coherence Tomography (IN• OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN• OCT consensus. Ophthalmology. 2014; 121: 1572-1578.
- Al-Sheikh M, et al. Quantitative OCT angiography of the retinal microvasculature and the choriocapillaris in myopic eyes. Investigative ophthalmology & visual science. 2017; 58: 2063-2069.
- Tang J, et al. Pathology of macular foveoschisis associated with degenerative myopia. Journal of ophthalmology. 2010; 2010.
- Park S and YJ Lee. Nano-mechanical compliance of Müller cells investigated by atomic force microscopy. International journal of biological sciences. 2013; 9: 702.
- Margolis R and RF Spaide. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. American journal of ophthalmology. 2009; 147: 811-815.
- Ichibe M, et al. Retinoschisis in a highly myopic eye without vision impairment. Retina. 2004; 24: 331-333.
- Hayashi K, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010; 117: 1595-1611.e4.
- Shimada N, et al. Progression from macular retinoschisis to retinal detachment in highly myopic eyes is associated with outer lamellar hole formation. British Journal of Ophthalmology. 2008; 92: 762-764.
- Baba T, et al. Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. American journal of ophthalmology. 2003; 135: 338-342.
- Shiraki N, et al. Fovea-sparing versus standard internal limiting membrane peeling for myopic traction maculopathy: a study of 102 consecutive cases. Ophthalmology Retina. 2020; 4: 1170-1180.
- Ho T-C, et al. Long-term outcome of foveolar internal limiting membrane nonpeeling for myopic traction maculopathy. Retina. 2014; 34: 1833-1840.
- Kumagai K, et al. Factors correlated with postoperative visual acuity after vitrectomy and internal limiting membrane peeling for myopic foveoschisis. Retina. 2010; 30: 874-880.
- Yu D-Y and SJ Cringle. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Progress in retinal and eye research. 2001; 20: 175-208.
- Sakata K, et al. Relationship between macular microcirculation and progression of diabetic macular edema. Ophthalmology. 2006; 113: 1385- 1391.
- Chin EK, et al. Staging of macular telangiectasia: power-Doppler optical coherence tomography and macular pigment optical density. Investigative ophthalmology & visual science. 2013; 54: 4459-4470.
- Veverka KK, et al. Noninvasive grading of radiation retinopathy: the use of optical coherence tomography angiography. Retina. 2015; 35: 2400-2410.
- Seike C, et al. Reopening of macular holes in highly myopic eyes with retinal detachments. Retina (Philadelphia, Pa.). 1997; 17: 2-6.
- Gonvers M and R Machemer. A new approach to treating retinal detachment with macular hole. American journal of ophthalmology. 1982; 94: 468-472.
- Ripandelli G, et al. Retinal detachment associated with macular hole in high myopia: using the vitreous anatomy to optimize the surgical approach. Ophthalmology. 2004; 111: 726-731.
- Mitamura Y, S Takeuchi and M Tsuruoka. Macular buckling combined with pars plana vitrectomy for complicated retinal detachment due to macular hole. Retina. 2000; 20: 669-672.
- Rouhette H, et al. Retinal detachment due to macular holes in highly myopic eyes. Prognostic factors. Journal francais d’ophtalmologie. 2001; 24: 49-53.
- Haut J, et al. Treatment by internal tamponade of retinal detachment with a macular hole. Journal francais d’ophtalmologie. 1987; 10: 707-715.
- Meng L, et al. Treatment of retinal detachment secondary to macular hole in highly myopic eyes: pars plana vitrectomy with internal limiting membrane peel and silicone oil tamponade. Retina. 2014; 34: 470-476.
- Nishimura A, et al. Efficacy of primary silicone oil tamponade for the treatment of retinal detachment caused by macular hole in high myopia. American journal of ophthalmology. 2011; 151: 148-155.
- Kadonosono K, et al. Treatment of retinal detachment resulting from myopic macular hole with internal limiting membrane removal. American journal of ophthalmology. 2001; 131: 203-207.
- Matsumura N, Y Ikuno and Y Tano. Posterior vitreous detachment and macular hole formation in myopic foveoschisis. American journal of ophthalmology. 2004; 138: 1071-1073.
- Abdelkader E and N Lois. Internal limiting membrane peeling in vitreo-retinal surgery. Survey of ophthalmology. 2008; 53: 368-396.
- Christensen UC, et al. Value of internal limiting membrane peeling in surgery for idiopathic macular hole stage 2 and 3: a randomised clinical trial. British Journal of Ophthalmology. 2009; 93: 1005-1015.