
Research Article
J Ophthalmol & Vis Sci. 2022; 7(1): 1065.
Tear Proteomic Approach and Identification of Tear Film Biomarkers with Rigid Gas Permeable Scleral Contact Lens (ScCLs) Wear for Keratoconus
Sah RK¹, Sharma N², Kaur J³, Sinha R², Vanathi M², Karmakar S4* and Titiyal JS*
1Scientist, Dr. R P Centre, AIIMS, Ansari Nagar, New Delhi, India
2Professor of Ophthalmology, Dr. R P Centre, AIIMS, Ansari Nagar, New Delhi, India
3Professor, Department of Ocular Biochemistry, Dr. R P Centre, AIIMS, Ansari Nagar, New Delhi, India
4Additional Professor, Department of Biochemistry, All India Institute of Medical Sciences, New Delhi, India
5Chief & Professor of Ophthalmology, Dr. R P Centre, AIIMS, Ansari Nagar, New Delhi, India
*Corresponding author: Subhradip Karmakar, Additional Professor, Department of Biochemistry, All India Institute of Medical Sciences, New Delhi, India
Jeewan S. Titiyal, Padma Shri Awardee, Chief & Professor of Ophthalmology, Dr. R P Centre, AIIMS, Ansari Nagar, New Delhi-110029, India
Received: February 25, 2022; Accepted: March 17, 2022; Published: March 24, 2022
Abstract
Keratoconus (KC) is a classical non-inflammatory disorder associated with elevated serum levels of IgE, IgG, and IgM [1,2]. Lema et al. [3] have described specific cytokines and protease profiles in patients with keratoconus; and found levels of IL-6, TNF-alpha, and MMP-9 were elevated in keratoconus subjects as compared to normal. The differential expression of tear film proteins such as MMP-1, keratins, and mammaglobin B can be found in keratoconus subjects [4]. Tear fluid has been successfully used as a source of biomarkers in several well-studied eye diseases. Studies performed on tear fluid in patients of keratoconus provided insights into the pathology of the disease and revealed probable prognostic and diagnostic biomarkers for the disease [5]. A multi-omics approach integrating data from proteomics, lipidomics and metabolomics is the need of the hour for studying tear fluid as an important source of biomarkers in keratoconus to lead to effective prognosis and treatment of the disease.
Tear fluid is a highly complex chemical and biological mixture containing proteins, peptides, electrolytes, lipids, and metabolites. It is proposed to be a surrogate representative for many eye diseases. It is an integral part of the ocular surface and represents the extracellular matrix for ocular surface epithelial cells [4,6]. Nano-mass Spectrometry and Liquid Chromatography were used in tear analysis, including analysis in proteomics, metabolomics, and lipidomics. Dry eye and meibomian gland dysfunction diseases involve the disruption of the lacrimal functional unit, resulting in symptoms of discomfort, visual disturbance, and tear film instability. These illnesses may exist independently as either symptomatic or asymptomatic disorders. However, they are frequently found in the same patient. The progression of these diseases typically leads to alterations in the tear film, tear hyperosmolarity, and the secretion of inflammatory mediators into the tears, initiated by cytokine release and metalloproteinase activation. Ocular dryness disorders also promote squamous metaplasia, mainly as a consequence of inflammation, whose severity can be used for grading different diseases affecting the ocular surface. In this study, we explored the efficacy of Rigid Gas Permeable Scleral Contact Lens in keratoconus regarding the expression of inflammatory Mediators.
Keywords: Tear Proteins & Tear Biomarkers; Cytokines and Interleukin Gene Ontology (GO); Keratoconus (KC); Ocular Surface Diseases (OSD); Liquid Chromatography-Mass Spectrometry (LC-MS/MS); Scleral Contact Lenses (ScCLs)
Introduction
Mass spectrometry has evolved tremendously since Professor Klaus Biemann [7] first analyzed amino acids in a mass spectrometer in 1958. The clear challenge in Biemann’s first experiment was how to introduce nonpolar molecules into the mass spectrometer to create ions which were eventually addressed with several new ionization techniques and sample introduction methods. Proteomics is the study of the proteome, the protein complement of the entire set of the genome. The terms “Proteomics and Proteome” were coined by Wilkins MR et al. [8] in the early 1990s and mirror the terms “Genomics and Genome”, which describe the entire collection of genes in an organism. These “Omics” terms symbolize a redefinition of how we think about biology and the workings of living systems. Tear Proteomics has been an essential revolutionary source of information in understanding ocular physiology. It can provide valuable insights into certain ocular surface diseases such as keratoconus & dry eye disease and is widely gaining popularity. The composition of the tears can reflect the state of inflammation or ocular surface damage, involving tear proteins such as inflammatory mediators. Tear fluid is chemically complex. It is proposed to be a surrogate representative for many eye diseases. Tear film analysis detecting changes in the levels of proteinases and cytokines has helped researchers gain a better understanding of the pathophysiology of complications. The majority of these proteins in the normal tear film consist of lysozyme, lactoferrin, secretory immunoglobulin, carbohydrate catabolism, proteolysis, proteases & protease inhibitors, protein transport serum albumin, lipocalin & lipophilin besides immune response, and regulation of apoptosis. A specific molecular signature from tear fluid analysis can help understand the etiology of the disease and help in prognosis. The tear fluid can serve as an optimal source of molecular targets for treating ocular disease conditions. The tear proteomics & protein biomarkers should ultimately start to help further determine the roles of these proteins in the etiology of keratoconus. The tear fluid has been an important source of information in our understanding of ocular physiology [9], providing valuable insights into certain ocular surface diseases such as dry eye disease. The composition of the tears can reflect the state of inflammation and correlate with disease severity [10,11]. Studies have shown that proteins such as matrix metalloproteinases, cytokines, and chemokines are present in human tears [12-14] orchestrating the immune response and regulation of apoptosis. Disease-specific molecular signature from tear fluid analysis can help understand the etiology of the disease and help in prognosis. In addition, tear fluid can serve as an optimal source of molecular targets for treating ocular disease conditions [11].
In this current study, we aimed to detect tear-film-based differential protein expression in keratoconus patients between cases (Wearing Scleral Contact Lens Eye) and controls (Non-wearing Scleral Contact Lens Eye). The expected outcome of our study will be helpful in determining the roles of these proteins in the etiology of keratoconus. The increasing need for advanced solutions for severe ocular surface disorders has fuelled technological advancements including the development of highly oxygen permeable materials and the evolution of contact lens designs. These lenses are used as therapeutic devices and provide effective protection of the ocular surface because of their distinct properties. It becomes an important tool to reduce the complications and the incapacitating symptoms produced by ocular disorders. The modern scleral lenses are unique in their design & highly oxygen permeable polymer to treat a variety of eye conditions, many of which do not respond to other forms of treatment. These lenses available today have the potential to really improve the lives of the patients not only by helping them see better but by providing all-day comfort. Tear film proteomics offers powerful analytical tools for studying the proteins involved in ocular diseases. Using these tools, we might be able to establish the precise functions of these proteins in the underlying pathophysiological processes and provide diagnostics biomarkers. The studies of differential protein expression in complex bio-fluids such as tear film require rapid, highly reproducible, and accurate quantification as well as to monitor the response of the patients to the scleral contact lens.
Material and Methods
See Design 1.
Design 1: Proteomics Work Flow.
Methods
The nano-Mass Spectrometry (ESI-QUAD-TOF SCIEX 5600) at Regional Centre for Biotechnology (RCB), Faridabad, Haryana had analyzed and provided both qualitative and quantitative informatics data for major tear proteins in our sample that are useful in assessing the health of the ocular surface.
Patient selection
All tear samples were collected from the Contact Lens Services, Dr. R P Centre for Ophthalmic Sciences, AIIMS, New Delhi, prior to Rigid Gas Permeable Scleral Contact Lens (SCL) Trail (Day=0) and then after dispensed SCL, the samples were collected at 1-month, 3-months, 6-moths, 9-months, and 12-months.
Tear sample collection
Approximately 4-6 μl of tear was collected from both eyes (one case eye & another control eye of the same patient) from each subject using calibrated hematocrit glass microcapillaries (Cat No.-T10H09), Mfg: Top Tech Lab Equipment Pvt. Ltd., India. The tear was collected from the inferior temporal side without anesthesia, minimizing ocular surface or lid margin irritation. Patients were instructed not to move their eyes or frequently close their eyelids during insertion of the pointed object (capillary tube) into the eye. After a successful collection, the tear samples of microcapillaries glass were gently placed into the pre-marked microcentrifuge tubes and stored at -80°C to prevent protein degradation until analysis.
Sample processing
The samples from the same patient (baseline, 1-month, 3-month, 6-month, 9-month, and 12-month) were pooled (n=5) in each subgroup of the case (Wearing Scleral Contact Lens Eye) and control (Non-wearing Scleral Contact Lens Eye) of the same patient. All the samples were taken out from -800C and were kept on ice. During analysis, all tear samples were pushed out from the microcapillaries tubes into microcentrifuge tubes through air pressure using tips of pipettes. The samples were combined with 20μl of a reagent consisting of 100mM Triethylammonium Bicarbonate (TEAB) buffer (pH 7.4). The blank capillaries were discarded. All the microcentrifuge tube with tear samples were centrifuged at 5000 rpm and stored at -80°C for later analyses.
Protein quantification
Proteins were quantitated using BCA (bicinchoninic acid) method (Thermo Scientific BCA Protein Assay Kit) as per the manufacturer’s protocol. A prior regression analysis was done with standard protein samples using BSA (bovine serum albumin) as standard. The regression lines were drawn through the origin and X, Y coordinates using the best fit average method. The correlation achieved was 95% (0.95). All test samples were estimated for proteins using the standard formula:
Ct/Cs=At/As, Ct=Concentration of Test, At=Absorbance of Test, Cs=Concentration of Standard, As=Absorbance of Standard
Sample preparation for mass spectrometry
75μg of protein was taken from each sample vial, and 200μL chilled acetone was added at a ratio (1:3). The samples were kept at -200C for 2hrs (2-12hrs). The samples were then centrifuged at 15000 rpm/40C for 20-minutes, and the supernatants were discarded, followed by a dry vacuum centrifuge (if required) to remove all the acetone from the samples. 20μL of 500mM TEAB was added to each sample tube and suspended. After this process, all samples were vortex mixed for 1-2 minutes and were then centrifuged at 5000 rpm for 2-minutes, followed by typsinization and mass spectrometry analysis.
Mass spectrometry
The workflow in a typical proteomics experiment involves sample fractionation followed by typsinization, peptide extraction, and MS/ MS analysis. The MS/MS sequence data were processed using a search algorithm MASCOT Distiller and MASCOT version 2.3.02 (Matrix Science, London, UK) search engine to obtain a peak list against the National Center for Biotechnology Information (NCBInr) database using only Homo sapiens (Human) entries.
Results
The common normalization technique was used on MS data requires that the data is transformed from the linear to the log scale. Doing so allows the values to conform to the normal distribution and reduces the likelihood of masking more relevant proteins with less relevant ones. Proteomic data obtained from mass spec from case and control were analyzed to sort the target proteins into different categories. These were used to analyze the candidate proteins into different classes as per their function, localization, or molecular pathway. Grouping of genes was based on functional similarity to enhance biological interpretation of large lists of genes systematically.
MMPs control & case: Heatmap and violin plot
MS results initially showed an elevated MMP in the test groups that returned to baseline upon a few days/months of SCL usage, indicating that it is well tolerated. Interestingly, an elevated Matrix Metalloproteinases (MMPs) in cases is also associated with increased tissue inhibitors of matrix metalloproteinases (TIMPs) showing a delicate interplay of balance between them (Figure 1A-1C).
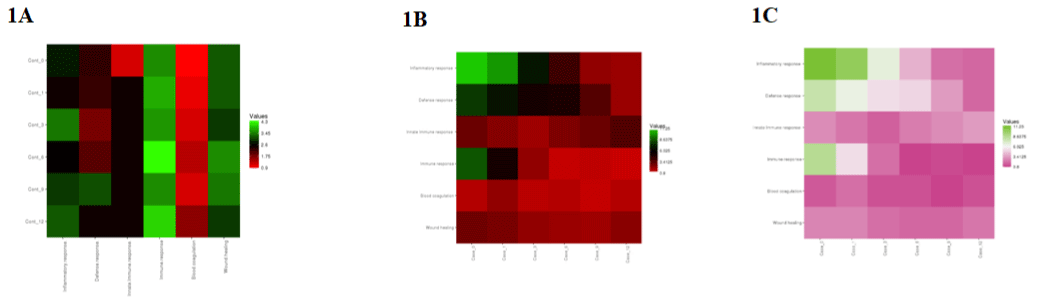
Figure 1: A) Showing enrichment of proteins from different GO categories in the control (untreated) samples. Tear proteomics were performed from subjects with
KC aided w/wo scleral contact lens and were investigated for changes in proteome profile. Subjects were followed longitudinally from time 0 to 12 months. Results
(Figure 1B and IC) show an enhanced immune response in KC (bi-lateral KC) with relatively less severe eye (Figure 1A). Heat map were generated using standard
software.
Proteomic profile of tear fluid
The proteins in pooled tear fluid samples from patients with keratoconus consisted of 10 eyes of 05 patients (Case: n=05 eyes) and (Control: n=05 eyes) were analyzed by liquid chromatography. In total, 435- proteins were identified in the tear fluid with a false discovery rate of less than 1% at the protein and peptide spectrum match levels, respectively. Out of which, we included 186-unique proteins with high confidence in fluid tear samples. Among the identified proteins in tear fluids, an average of 80-90% proteins was common between case and control, indicating that most of the proteins in the case were detected in control. Pairwise analysis of control and case as represented by illustrated the different enriched pathways (Figure 2 and 3). Figure 3A and 3B shows an enriched immune-related pathway associated with inflammatory response, defense response, blood coagulation, wound healing, and response to foreign material. We also identified the immune set genes associated with KC as evident by the vertical box plots. The results show a dynamic variation in the proteome profile that exhibits a gentle surge at 1M (1-Month) in the test which normalizes to the baseline in advancing months. We do, however also see a variation in commonly occurring proteins between case and control across different months. In addition, after a year, proteins related to immune response, inflammation, and matrix remodeling were not found in the case that was initially identified in tear fluids at baseline. Therefore, these proteins originated from the ocular surface. Further, a violin plot representation for the same shows the date distribution along with its usual contour that seems to exhibit an initial enhancement of a few GO pathways in case 0M (baseline) as compared to control 0M (baseline) (Figure 4). To determine the cellular components of tear fluids proteins, we conducted gene ontology (GO) analysis using a database for annotation, visualization, and integrated discovery (version 6.7). The tear fluids of the cellular pool are comprised of a protein belonging to different gene ontology (GO) categories like extracellular region protein, catalysis, immune response, and matrix remodeling. Our analysis showed that proteins belonging to different GO categories were distributed differently between cases and controls, suggesting dynamics alternations in proteins.
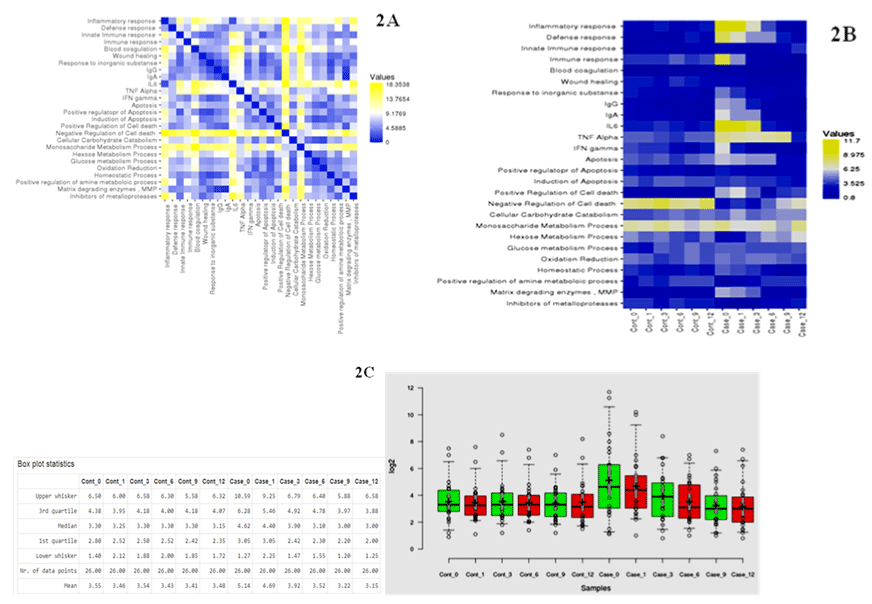
Figure 2: A) Heatmap representation showing pairwise comparison between controls and cases. B) The different GO categories were displayed that showed
enrichment in MS/MS. The absolute coordinates were plotted in two dimensions (x, y). The pairwise distance program treats each row of data as a spatial
coordinate; calculate distance between all pairs of coordinates. C) Box Plot showing differential enrichment and dynamic change in proteomic profiling of the cases
vs. controls. Results show a shuttle variation in proteome profile in cases initially which after intervention resulted in gradual return to base line.
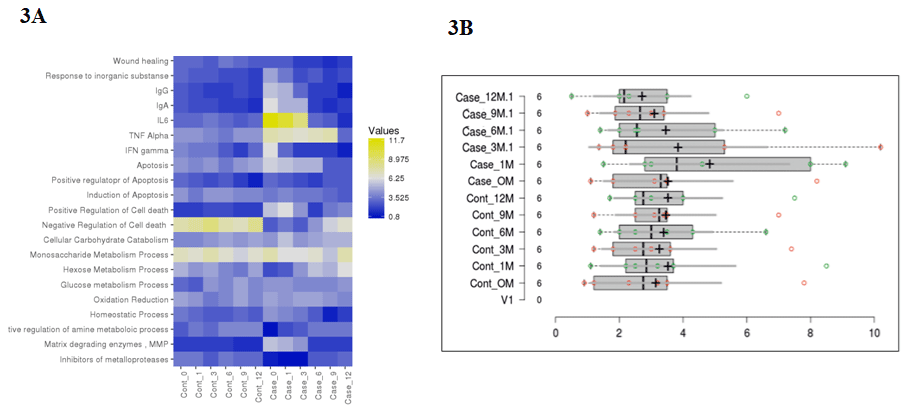
Figure 3: A) Showing enrichment in immune and matrix remodeling pathway in the cases (treated) samples and controls (untreated). Quantitative proteomics
performed after mass spectrometry. Results show increased immune system activation (yellow grids) as well as matrix degradation activity in case 0M and 1M
following which inflammation slows down and normalizes after few months. B) Histogram representing the same.
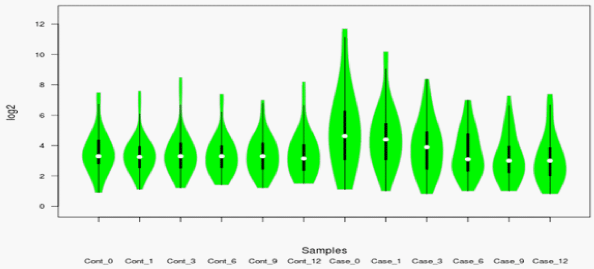
Figure 4: Violin showing differential enrichment and dynamic change in proteomic profiling of the cases vs. controls. Results show a shuttle variation in proteome
profile in cases initially which after intervention resulted in gradual return to base line.
Differentially expressed proteins in tear fluid sample of patients with keratoconus
We found about 85-proteins are differentially expressed proteins in tear fluids, although there seems to be a dynamic variation of the proteome profile across different time points. A heatmap analysis grid represents in (Figure 2). Among the proteins, inflammatory biomarkers were moderately up-regulated, and anti-inflammatory signatures were down-regulated in the tear fluid of keratoconus patients (Figure 3). Furthermore, IL6, TNF alpha, Interferon-gamma expression levels were increased but reduced in TGF beta1 in tear fluid of keratoconus patients compared to controls (Table 1).
Proteins
Expressions (Up-regulated or Down-regulated)
Source
Interleukin (IL-6)
Up-regulated (↑)
Tear Fluid
Tumor Necrosis Factor (TNF-alpha)
Up-regulated (↑)
Tear Fluid
Interferon (IFN-gamma)
Up-regulated (↑)
Tear Fluid
Transforming Growth Factor (TGF beta1)
Down-regulated (↓)
Tear Fluid
Matrix Metalloproteinase (MMP-9)
Up-regulated (↑)
Tear Fluid
Table 1: Summarized list of Major Tear proteins in Keratoconus.
Gene ontology (GO) enrichment analysis of keratoconus
One of the main uses of the GO is to perform enrichment analysis on gene sets. Gene Ontology (GO) term enrichment is a technique for interpreting sets of genes using the Gene Ontology system of classification, in which genes are assigned to a set of predefined bins depending on their functional characteristics. The functional role associated with each of the identified proteins in keratoconus from tear fluids will be compared to an already existing database of proteins of known function. We first compared the Gene Ontology & Biological Pathway (GOBP) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis using up and downregulated Differentially Expressed Proteins/Peptides (DEPs). In our proteome data, we have found the following gene ontology pattern of differentially expressed genes. The selected identified tear proteins of keratoconus are mentioned in (Table 2).
Sl No.
Name of Proteins
1
pHL E1F1
2
Proline Rich, Lacrimal 1
3
Unnamed Protein Product
4
Lacritin Precursor
5
Lysozyme {Beta-sheet domain} [Human, Peptide Mutagenesis, 130 aa]
6
Chain A, Changes In Conformational Stability of A Series of Mutant Human Lysozymes At Constant Positions
7
Chain A, Contribution of Hydrophobic Effect To The Conformational Stability of Human Lysozyme
8
Chain A, Crystal Structure of Mutant Human Lysozyme Substituted At Left-Handed Helical Positions
9
Lysozyme Precursor (EC 3.2.1.17)
10
Chain A, Role of Amino Acid Residues At Turns In The Conformational Stability and Folding of Human Lysozyme
11
Chain A, Crystal Structure of Mutant Human Lysozyme Substituted At The Surface Positions
12
Hypothetical Protein LOC401137
13
Serum Albumin
14
Apolipoprotein J Precursor
15
Lactoperoxidase isoform 1 Preproprotein
16
Lipocalin 1 Precursor
17
Chain A, X-Ray Crystal Structure of Human Ceruloplasmin At 3.0 Angstroms
18
Galectin 3 binding Protein
19
Peptide PB,Saliva
20
Pre-pro-megakaryocyte potentiating factor
21
Basement membrane-specific heparan sulfate proteoglycan core protein precursor variant
22
Heparan sulfate proteoglycan
23
Zn-alpha2-glycoprotein
24
Keratin 1
25
Transmembrane secretory component; poly-Ig receptor; SC [Homo sapiens]
26
Cystatin SA-III=potential precursor of acquired enamel pellicle [human, Peptide, 121 aa]
27
Proline rich 4 (lacrimal) isoform 2 [Homo sapiens]
28
Prolactin-induced protein [Homo sapiens]
29
Phospholipid transfer protein isoform a precursor [Homo sapiens]
30
HRPE773 [Homo sapiens]
31
Secretoglobin, family 2A, member 1 [Homo sapiens]
32
Nucleobindin 2 [Homo sapiens]
33
NUCB2 protein [Homo sapiens]
34
Gelsolin isoform a precursor [Homo sapiens]
35
Haptoglobin Hp2
36
Airway lactoperoxidase [Homo sapiens]
37
Lipophilin A precursor [Homo sapiens]
38
Cathepsin L1 preproprotein [Homo sapiens]
39
Lysyl hydroxylase
40
Cathepsin D preproprotein [Homo sapiens]
41
Osteosarcoma amplified 9 isoform 1 precursor [Homo sapiens]
42
Serine protease [Homo sapiens]
43
Ig A1 Bur
44
SNC66 protein [Homo sapiens]
45
MHC class I antigen [Homo sapiens]
46
MCH class I HLA-B*5104 [Homo sapiens]
47
human leucocyte antigen A [Homo sapiens]
48
RecName: Full=Histone H2B type F-S; Short=H2B.s; Short=H2B/s
49
Dermcidin preproprotein [Homo sapiens]
50
Transcobalamin I precursor
51
hCG201503 [Homo sapiens]
52
Cathepsin B Preproprotein
53
Tripeptidyl-peptidase I Preproprotein
54
Chain C, Crystal Structure of Mt-Sp1 In Complex With Fab Inhibitor E2
55
Cystatin SN [human, submandibular-sublingual saliva, Peptide Partial, 35 aa]
56
CANT1 Protein
57
Hypothetical Protein [Trypanosoma brucei TREU927]
58
Immunoglobulin Kappa light chain variable region
59
Immunoglobulin Kappa chain variable region
60
Anti-rabies virus immunoglobulin light chain variable region
61
Anti-tetanus toxoid immunoglobulin light chain variable region
62
Immunoglobulin light chain variable region EM1-PPS-4-K1-5
63
Immunoglobulin Kappa light chain
64
Chain A, Nmr Structure Of The N-Terminal Domain A of The Glycoprotein Chaperone Erp57
65
Chitinase
66
Nucleobindin
67
Actin, alpha 1, skeletal muscle
68
Ribonuclease 4, RNase 4 [human, blood plasma, Peptide, 119 aa]
69
KIAA0088
70
GRP78 precursor
71
RNA-binding protein YhbY [Salmonella enterica subsp. enterica serovar Typhi str. CT18]
72
Cystatin S acidic isoform [human, submandibular-sublingual saliva, Peptide Partial, 35 aa]
73
Proapolipoprotein
74
Ribonuclease T2 precursor
75
Cystatin D
76
HRV Fab 027-VL
77..
Anti-Toxoplasma gondii SAG1 immunoglobulin s2 light chain variable region
78..
Keratin 9
79
Biotinidase precursor
80
Glyceraldehyde-3-phosphate dehydrogenase
81
Dystroglycan 1 preproprotein
82
Mucolipin 2, isoform CRA_b
83
GalNAc-T5
84
Keratin type II
85
Actin type 2 [Marginopora kudakajimensis]
86
Alpha-1-antitrypsin
87
Alpha-1 antitrypsin
88
Cryptochrome 1 (photolyase-like)
89
Chain A, The Crystal Structure Of Cellular Repressor Of E1a- Stimulated Genes (Creg)
90
Unknown protein
91
DnaJ (Hsp40) homolog, subfamily C, member 3
92
Chain L, Structure of The B20-4 Fab, A Phage Derived Fab Fragment, In Complex With Vegf
93
Chain A, Crystal Structure of The Fab Fragment From The Monoclonal Antibody CetuximabERBITUXIMC-C225
94
Anti-HIV-1 gp120 immunoglobulin 412d kappa light chain
95
Immunoglobulin kappa light chain VLJ region
96
Glyceraldehyde-3-phosphate dehydrogenase A [Cupriavidus taiwanensis]
97
DENN/MADD domain containing 4B
98
Lipase-like protein [Trypanosoma brucei TREU927]
99
START domain containing 5, isoform CRA_b
100
Conserved hypothetical protein, Metallo-hydrolase/oxidoreductase motif [Cupriavidus taiwanensis]
101
Alpha-fibrinogen precursor
102
Heat shock protein 27
103
IgE-binding protein
104
Threonyl-tRNA synthetase [Trypanosoma brucei TREU927]
105
Chain A, Crystal Structure of Cys10 Sulfonated Transthyretin
106
Hypothetical protein FLJ38723, isoform CRA_a
107
ERGIC53
108
2-phosphopyruvate-hydratase alpha-enolase; carbonate dehydratase
109
Hevin-like protein
110
hCG1811616
111
Neuraminidase precursor
112
Immunoglobulin lambda light chain VLJ region
113
Chain L, Rational Development of High-Affinity T-Cell Receptor-Like Antibodies
114
Immunoglobulin lambda 6 light chain
115
Chain C, Solution Structure of Human Immunoglobulin M
116
Anti-peptide/MHC complex HLA-A1/MAGE-A1 monoclonal antibody light chain
117
Protein NIG51 lambda,Bence-Jones
118
Mitogen-activated protein kinase kinase kinase 3 isoform 1
119
Glucosaminyl (N-acetyl) transferase 3, mucin type
120
Immunoglobulin gamma heavy chain variable region
121
Ribosomal protein L4
122
Activated p21cdc42Hs kinase
123
T-cell receptor beta chain
124
Hypothetical protein - human
125
S6 kinase b
126
Cofactor of BRCA1, isoform CRA_b
127
N-acetylglucosamine-6-O-sulfotransferase (EC 2.8.2.-) - human
128
Chain F, Structure of The Rgs-Like Domain From Pdz-Rhogef
129
pHL E1F1
130
Unnamed protein product
131
Chain A, Contribution of Hydrophobic Effect To The Conformational Stability f Human Lysozyme
132
Basement membrane-specific heparan sulfate proteoglycan core protein precursor variant
133
Tripeptidyl-peptidase I preproprotein
134
Leucine-rich alpha-2-glycoprotein 1
135
MHC class I antigen
136
Serine protease
137
Mucolipin 2, isoform CRA_b
138
Mucolipin 2, isoform CRA_b
139
Invasion associated protein p60 [Listeria monocytogenes]
140
Pre-mRNA splicing factor
141
FLJ00103 protein
142
DnaJ (Hsp40) homolog, subfamily C, member 3
143
Down Syndrome cell adhesion molecule isoform CHD2-42 precursor variant
144
MMP-9 (Matrix Metalloproteases)
145
TNF alpha (Tumor necrosisnfactor)
146
IL6
147
Collagen Type IV
Table 2: Showing the name of a few proteins identified by Mass Spectrometry in Keratoconus Tear Fluid.
Statistical analysis
Normalization techniques are used to remove systematic biases from peptide samples. These biases can arise from various sources including protein degradation, measurement errors, and variation in loading samples. The common normalization techniques used on MS data require that the data is transformed from the linear to the log scale. Doing so allows the values to conform to the normal distribution and reduces the likelihood of masking more relevant proteins with less relevant ones. Simca-P+12.0 was used to perform a principal component analysis (PCA) to rule out the ambiguity in the samples and to identify potential biases between patients to monitor a representative quantitative ratio distribution. Few data points were identified as an outlier and were binned out from the main analysis. The top canonical pathway identified is associated with immune pathways and matrix modeling.
Discussion
Keratoconus is associated with tissue degradation involving extracellular matrix remodeling, collagen deficiency [15], and increased roles of pro-inflammatory cytokines, cell adhesion molecules, and matrix metalloproteinases3. The differential gene expression analysis in corneal epithelia of patients with keratoconus showed dysregulations of immune pathways with an elevated expression of matrix metalloproteinase-9 (MMP9) and other inflammatory markers. Further, a positive correlation between the reduced collagen expressions with clinical severity of keratoconus was observed, indicating their role in structural deformity in this disease [15]. These genes were also associated with elevated inflammatory cytokine IL-6 in the corneal epithelium and in the tears of keratoconus patients. Although keratoconus doesn’t seem to have an inflammatory etiology, growing evidence suggests that subtle to moderate inflammation may be associated with the condition. Previous reports showed an elevated serum level of IgE, IgG, and IgM in keratoconus. Recently, Lema et al. [3] analyzed specific cytokines, cell adhesion molecules, and proteases in patients with keratoconus, and found an elevated level of IL-6, TNF-a, and MMP-9 in keratoconus subjects as compared to normal with MMP9 may be associated with corneal inflammation16. Thus, it may be concluded that keratoconus is an inflammatory disorder. Results from our study correlated with previous a report, which indicates that elevated expression levels of IL6, TNF alpha, Interferon-gamma along with a reduced expression in TGF beta1 is associated with positive immune response, as seen in tear fluid of KC patients immediately following scleral contact lens as compared to controls of keratoconus patients in the other eye. A gradual adaptation followed this as evident by a reduced immune response and an associated improvement in visual acuity. Collier SA et al. [17] postulated the involvement of MMPs and their possible role in keratoconus. The Interleukin-1 (IL-1) can induce MMP-9, and it’s known that keratoconus fibroblasts overexpress IL-1 receptors to normal18 and thus, the symptoms of keratoconus are an outcome of excess Interleukin-1 beta (IL-1β). Normally, the human cornea is 70% collagen by weight. In keratoconus, the thinning & ectasia are found mostly due to damage of extracellular matrix and a decrease in types I and IV collagen content has been reported due to the high collagenase activity. As studied by Abalian JH et al. [19]; levels of telopeptides of collagen degradation products were found to be higher levels in the tear film of keratoconus patients. Cristina Kenney M, et al. [20], described a cascade hypothesis of keratoconus in which enzymes could possibly lead to oxidative damage by altering corneal proteins and ultimately lead to apoptosis, altered signaling pathways, increased enzyme activities, and fibrosis. These cytotoxic agents in keratoconus corneas may lead to corneal thinning and loss of vision. This hypothesis is supported by evidence showing that the inhibitors of destructive enzymes alpha-one (a1) proteinase inhibitor, alpha-two (a2) macroglobulin, and tissue inhibitor metalloproteinase one (TIMP-1) are decreased in keratoconus corneas; the latter of which can inhibit cell apoptosis [21-23]. Cristina Kenney M et al. [20] guessed that reactive oxygen species may lead to large amounts of cytotoxic by-products in keratoconus corneas eventually leading to corneal thinning and loss of vision.
Corneal scarring is significant in keratoconus because it leads to a reduction in transparency and visual impairment. In a normal cornea, the epithelium is constantly renewed; however, layers such as the endothelium or basement membrane are compromised. A repair response is initiated by various growth factors and cytokines that contribute to fibrotic tissue. Transforming growth factorbeta (TGFβ) is important in ocular scar development in activating macrophages, corneal fibroblasts, and other fibrosis-related growth factors [24]. Excessive deposition of fibrotic tissue in the cornea can lead to pathologies involving excessive scarring and contracture. The fibrotic repair causes disorganization of the fibrotic tissue affecting both transparency and shape of the corners [25]. We are currently undertaking extensive proteome work to identify the tear fluid proteomics that seems too perturbed in KC and its correlation with disease etiology. This study carried out the detailed tear protein profiling of keratoconus patients using a rigid gas-permeable scleral contact lens and identified a comparative quantity of proteins between different follow-ups. Many proteins were up & downregulated in both groups. This enabled a comparison of the two group patients between case & control and their impact on selected proteins at 0-day along with 5-consecutive follow-ups. Tear protein profile in keratoconus may result in tear film instability, aqueous deficiency, and increased tear hyperosmolarity. Keratoconus is associated with tissue degradation that involves extracellular matrix remodeling, collagen deficiency, and more recently, increased roles of pro-inflammatory cytokines, cell adhesion molecules, and matrix metalloproteinase. These genes were also associated with elevated inflammatory cytokine IL-6 in the corneal epithelium and in the tears of keratoconus patients. Although keratoconus doesn’t seem to have an inflammatory etiology, growing evidence suggests that subtle to moderate inflammation may be associated with the condition. Previous study reports showed an elevated serum level of IgE, IgG, and IgM in keratoconus. Thus, it may be concluded that keratoconus has a distinct inflammatory signature. Our study results correlated with previous studies and indicated that IL6, TNF alpha, Interferongamma expression levels were increased but reduced in TGF beta1 in tear fluid compared to controls of keratoconus patients. At this point, with our limited data, we are however unable to comment on whether keratoconus is the cause or result of inflammation. Furthermore, it is important to note that our significant results were observed in larger sample sizes of keratoconus patients, suggesting high external and internal validity. Future research may be conducted to extend the results of the present study regarding tear proteomics and tear fluid biomarkers on a larger sample size and for longer follow-up periods.
Conclusions
The initial tear protein analysis indicates that keratoconus may be associated with the differential expression of several proteins. As a source of biomarkers in ocular and systemic conditions, the tear protein has been well studied and is shown to have translational potential. The non-invasive way of collecting the tear fluid makes it an optimal biological fluid to study with minimal to no discomfort to the patient. Our study indicates that keratoconus is associated with an inflammatory response that showed an elevated level of cytokines and tissue degrading enzymes. This study results followed longitudinally over one year time, binned at regular intervals showed a transient surge in the inflammatory response following scleral lens (case arm) indicative of the natural immune response by the ocular surface. Violin plot shows a slow adaptation and a reduction in inflammation associated with a significant improvement and adaptation by the ocular surface. Interestingly, our observations agree well with the clinical observations showing marked improvement in vision and comfort of the patients.
Acknowledgement and Disclosure
Ethical Clearance: Institutional ethical clearance was obtained prior to the study and sample collection with consent from the patients.
Authors’ Contribution: RKS performed the study with assistance from Co-authors. JS and SK oversaw the work.
References
- Kemp EG, Lewis CJ. Immunoglobulin patterns in keratoconus with particular reference to total and specific IgE levels. Br J Ophthalmol. 1982; 66: 717-720.
- Rahi A, Davies P, Ruben M, Lobascher D, Menon J. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977; 61: 761-764.
- Lema I, Durán JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005; 112: 654-659.
- Catherine P, Heather LC, Jason JN. Tear proteomics in Keratoconus. Molecular Vision. 2010; 16: 1949-1957.
- Krishnatej Nishtala, Natasha Pahuja, Rohit Shetty, Rudy MMA Nuijts, Arkasubhra Ghosh. Tear biomarkers for keratoconus. Eye Vis (Lond). 2016; 3: 19.
- Acera A, Vecino E, Rodriguez-Agirretxe I, et al. Changes in tear protein profile in keratoconus disease. Eye (Lond). 2011; 25: 1225-1233.
- Biemann K. Mass Spectrometry: Organic Chemical Applications. New York: McGraw Hill Book Co., Inc. Republished. American Society for Mass Spectrometry: Classic Works in Mass Spectrometry. 1962; 1. New York: McGraw-Hill.
- Wilkins MR, Sanchez JC, Gooley AA, Appel RD, Humphery-Smith I, Hochstrasser DF. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. 1996; 13: 19-50.
- Pieragostino D, D’Alessandro M, di Ioia M, Di Ilio C, Sacchetta P, Del Boccio P. Unraveling the molecular repertoire of tears as a source of biomarkers: Beyond ocular diseases. Proteomics Clin Appl. 2015; 9: 169-186.
- Huang JF, Zhang Y, Rittenhouse KD, Pickering EH, McDowell MT. Evaluations of tear protein markers in dry eye disease: repeatability of measurement and correlation with disease. Invest Ophthalmol Vis Sci. 2012; 53: 4556-4564.
- Von Thun Und Hohenstein-Blaul N, Funke S, Grus FH. Tears as a source of biomarkers for ocular and systemic diseases.Exp Eye Res. 2013; 117: 126-137.
- Fodor M, Kolozsvari BL, Petrovski G, Kettesy BA, Gogolak P, Rajnavolgyi E, et al. Effect of contact lens wear on the release of tear mediators in keratoconus. Eye Contact Lens. 2013; 39: 147-152.
- Huang D, Xu N, Song Y, Wang P, Yang H. Inflammatory cytokine profiles in the tears of thyroid-associated ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 619-625.
- Poyraz C, Irkec M, Mocan MC. Elevated tear interleukin-6 and interleukin-8 levels associated with silicone hydrogel and conventional hydrogel contact lens wear. Eye Contact Lens. 2012; 38: 146-149.
- Shetty R, Sathyanarayanamoorthy A, Ramachandra RA, Arora V, Ghosh A, Srivatsa PR, et al. Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity. Mol Vis. 2015; 21: 12-25.
- Li DQ, Pflugfelder SC. Matrix metalloproteinases in corneal inflammation. Ocul Surf. 2005; 3: S198-202.
- Collier SA. Is the corneal degradation in keratoconus caused by matrixmetalloproteinases? Clin Exp Ophthalmol. 2001; 29: 340-344.
- Fabre EJ, Bureau J, Pouliquen Y, Lorans G. Binding sites for human interleukin-1 alpha, gamma interferon and tumor necrosis factor on cultured fibroblasts of normal cornea and keratoconus. Curr Eye Res. 1991; 10: 585- 592.
- Abalain JH, Dossou H, Colin J, Floch HH. Levels of collagen degradation products (telopeptides) in the tear film of patients with keratoconus. Cornea. 2000; 19: 474-476.
- Cristina Kenney M, Brown DJ. The cascade hypothesis of keratoconus. Cont Lens Anterior Eye. 2003; 26: 139-146.
- Zhou L, Sawaguchi S, Twining SS, Sugar J, Feder RS, Yue BY. Expression of degradative enzymes and protease inhibitors in corneas with keratoconus. Invest Ophthalmol Vis Sci. 1998; 39: 1117-1124.
- Brown DJ, Lin B, Chwa M, Atilano SR, Kim DW, Kenney MC. Elements of the nitric oxide pathway can degrade TIMP-1 and increase gelatinase activity. Mol Vis. 2004; 10: 281-288.
- Sawaguchi S, Twining SS, Yue BY, Wilson PM, Sugar J, Chan SK. Alpha-1 proteinase inhibitor levels in keratoconus. Exp Eye Res. 1990; 50: 549-554.
- Saika S, Yamanaka O, Sumioka T, Miyamoto T, Miyazaki K, Okada Y, et al. Fibrotic disorders in the eye: Targets of gene therapy. Prog Retin Eye Res. 2008; 27: 177-196.
- Fini ME, Stramer BM. How the cornea heals: Cornea-specific repair mechanisms affecting surgical outcomes. Cornea. 2005; 24: S2-S11.