
Research Article
J Ophthalmol & Vis Sci. 2023; 8(1): 1072.
Perspiratio Insensibilis of the Cornea
Schrage T1, Panfil C1,2, Fuest M1, Schrage N1 and Urbach M3
1Aachen Centre for Technology Transfer in Ophthalmology, Karlsburgweg 9, Germany
2Clinic for Ophthalmology, RWTH Aachen University Hospital, Pauwelsstrasse 30, Germany
3ACTO Service GmbH, Karlsburgweg 9, Germany
*Corresponding author: Thomas Schrage Centre for Technology Transfer in Ophthalmology, Karlsburgweg 9, D 52070 Aachen, Germany
Received: November 16, 2022; Accepted: December 26, 2022; Published: January 02, 2023
Abstract
Introduction: The deswelling of the cornea via the natural pathways of perspiratio insensibilis and endothelial pump has been known for a long time. However, reliable data on the amount of perspiratio insensibilis, especially in edematous corneas, have been lacking until now.
Material and Methods: In the Ex Vivo Eye Irritation Test (EVEIT), corneas can be observed purely physically under defined and stable biochemical conditions. The thickness and temperature of the corneas is measured by infrared thermography and Optical Coherence Tomography (OCT).
Three corneas in stable MEM EVEIT culture and three corneas with induced and reversible edema in an EVEIT osmolar deficiency culture were placed in at 20°C and 60% humidity while tracking thickness and temperature for 30 minutes.
Results: Over a period of 30 minutes, the temperature of both, healthy and edematous EVEIT corneas decreased from an initial 28°C to 21°C. The corneal thickness decreased by 51 ±12 μm in the healthy corneas and by 131 ±16 μm in the edematouscorneas.
Conclusion: The considerable thickness differences between healthy and swollen corneas might give a physical and physiological explanation of some observable clinical effects in Fuchs’s endothelial dystrophy. The clinically observed effect of clearing of the cornea during the waking period. Furthermore, some phenomena of Dry Eye Syndrome (DES) can be explained by this difference of increased water release from the eye.
Keywords: Cornea; Dry Eye Syndrome; Perspiratio Insensibilis; Fuchs’s endothelial dystrophy.
Introduction
How does the cornea lose its water? This fundamental question on the homeostasis of the cornea is investigated in this project. The corneal water content is about 70-80% (Kampa et al. 2002) [1]. This specific hydration is crucial for the optical transparency of the corneal stroma (Ehlers et al. 1966) [2]. Furthermore, there is a need of the correct composition of collagens and proteoglycans. Under physiological conditions of closed epithelium with tight junctions water mainly enters the corneal stroma via the endothelium, very little amounts of additional water derive from tears and from the scleral base. As soon as the pressure in the eye increases or any layer of the cornea become dysfunctional, the corneal water starts to grow up (Ehlers et al. 1967) [3]. The former research found that semi-permeable layers and the active transport of water are essential for maintaining corneal transparency (Kuerten et al. 2015) [4]. Corneal opacities with intact active transport of the endothelium are typically caused by endothelia injuries which result in an increased water inflow into the corneal stroma. The same mechanism combined with intra-epithelial edema can be seen in acute glaucoma when the intraocular pressure is >= 70 mmHg and water is physically pressed into the corneal stroma (Ehlers et al. 2004) [5]. This becomes obvious when the intraocular pressure exceeds 70 mmHg (Ytteborg et al. 1965) [6]. Another way of corneal hydration imbalance is, finally, the opening of the scleral entry port and submersion in MEM for corneal grafts in the European corneal culture system, which leads to considerable swelling of the corneas with the need of deswelling (Lindstrom et al. 1990) [7] before grafting either with dextran (Reim et al. 2008) [8] or as in the American system, where chondroitin sulphate is added
Our objective in this objective was to find out, to which extend passive perspiration is part of the deswelling of the cornea in real life. Clinically, ophthalmologists are familiar with patients suffering from corneal edema in Fuchs’ dystrophy have worse symptoms in the morning after a sleeping period. The gradual loss of the metabolically active endothelium reduces the active water transport from the corneal stroma (Adamis et al. 1993) [9]. After a sleeping phase with closed lids, stopping any perspiratio insensibilis the diseased cornea at morning eye opening is swollen. During the day, in these cases vision slowly improves to be best and stable after noon. One of the open questions is in which extend this is caused by the additional loss of water from evaporation from the cornea, or if other factors of daytime change the opacity of the corneas. To answer this, we try to quantify the influence of perspiratio insensibilis on the water loss of the cornea. This is particularly interesting to gain a better understanding of the mechanism of Fuchs’ dystrophy and as well also for the dry eye disease as a hyper-evaporative disease.
Material and Methods
In the Ex Vivo Eye Irritation Test (EVEIT), we expose the cultured corneas in an air lift system to various chemical and physical stimuli while providing and monitoring vital conditions by using high-resolution optical coherence tomography (OCT) (Pinheiro et al. 2015) [10]. We added in this study thermography to this set of quantitative measurements.
Perspiratio insensibilis is defined as the loss of water due to evaporation from skin, mucous membranes, alveolar surfaces, and other surfaces. In this article we try to quantify the loss of water over the cornea measured by the loss of thickness [11].
An infrared camera (Seek-Thermal (Seek Thermal, Inc. 6300 Hollister Ave Santa Barbara, CA 93117.), which is controlled via an i-Phone12 mini (Apple, Apple, One Apple Park Way, Cupertino, CA 95014) was used for the thermography. For the touch free measurement of corneal thickness, we used an OCT from Thorlabs. Device: Ganymed Spectral Reader, Thorlabs HL GmbH, Münchner Weg 1, Bergkirchen, Germany).
Healthy EVEIT Cornea in Standard Culture
We took 3 rabbit corneas directly from the animal slaughter and cultured them within 4 hours. The eyes were pre-cultured in serum-free culture with Minimal Essential Medium (MEM) in the EVEIT chamber system and treated for 24 h in the incubator under EVEIT culture conditions. Subsequently, the thickness of the corneas was measured by OCT. For vitality and quality control a glucose lactate measurement in the culture medium was performed. This must contain minimum criteria of more than 2 mmol glucose/l and more than 1.5 mmol lactate/l (measured withGOD-PAP and LOD-PAP; Greiner Diagnostic GmbH, Bahlingen, Germany) in the medium passed through the chamber to work scientifically with the corneas. Furthermore, the corneal surface was stained with sodium fluorescein and illuminated with cobalt blue light to demonstrate a fully intact epithelium. Complete transparency of the cornea in transmitted light was performed to complete this clinical quality control. The exact conditions have been published previously (Frentz et al. 2008) [12].
Corneal Edema in the EVEIT Cornea by Osmolar Deficient Culture Medium
In another experiment, we cultured 3 additional EVEIT rabbit corneas that were vital and fully epithelialized as described above. After 24 hours in the regular medium, we changed the MEM with a hypoosmolare swelling medium (MEM + 0.3% saline solution 1:4). According to the experiments by Panfil et al., these corneas (Dutescu et al. 2015) [13] showed uniform increase in corneal thickness under culture conditions in the incubator at 32°C temperature and 99% humidity.
Sequence of Measurements
All corneas were removed from the incubator at 99% humidity and 32°C and immediately covered with metal lids to maintain temperature and vapor pressure stable for a short time. When all measuring equipment was organized after less than 2 minutes the covers were removed, and the thickness of the corneas was measured by OCT. During OCT measurements the temperature of the apex corneae was measured at 20°C room temperature and 60% humidity by infrared thermography camera.
Both sets of measurement were performed in intervals of 6 minutes. This measurement set-up enables a temperature / corneal thickness diagram (Figure 5).
Results
For healthy corneas under normal culture medium, there is a maximum change in thickness of 51.1 μm (Figure 3). Figure 4 shows a considerable swelling of the corneal stroma to thicknesses around 760 μm (±58 μm) in EVEIT corneas in the deficiency medium culture. While the corneal temperature drops from 28°C to 21°C as in healthy corneas, the corneal thickness changes for a total of -138 μm (±16)over 30 minutes at 20°C room temperature. There seems to be a considerable difference in evaporation for healthy and swollen corneas. Computing the volume change we see -0.00578 ml in healthy and -0.015 ml in swollen rabbit corneas with a mean diameter of 12 mm. The difference is considerable and cannot be explained by temperature and vapor pressure which were similar. Under the same environmental conditions (temperature and humidity), the swollen corneas loose more than 2.5 times of the volume compared to healthy corneas in the same culture and condition. This shows that a swollen cornea is significantly more affected by evaporation than a non-swollen cornea.
Figure 1: Corneal edema in Fuchs’ dystrophy.
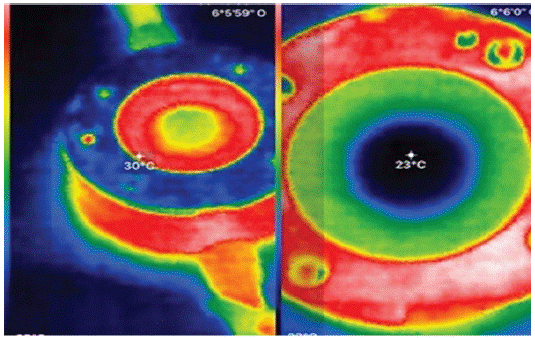
Figure 2: Ex vivo Eye Irritation Test (EVEIT) Chamber with rabbit
cornea, left directly after opening the metal lid Temperature of the
chamber 30°C of the apex corneae 28°C, right 15 minutes later with
clear cooling of the apex corneae to 23°C, the chamber itself is still
29°C (red ring).
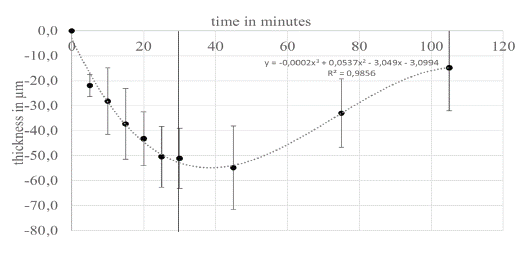
Figure 3: Healthy EVEIT cornea taken in culture (without swelling).
The cornea initially stands for 30 min at 20°C room temperature
and 60% humidity. The corneal thickness decreases continuously.
With a mean initial thickness of all corneas (n=3) of 558 μm (standard
deviation (Std) ±22 μm), the mean decrease over 30 min was
51.1 μm (Std±12 μm). At t>30 min the ambient temperature was
lowered to 9 °C and 40% humidity. This then leads to a significant increase
in corneal thickness if the surface continues to lack wetting.
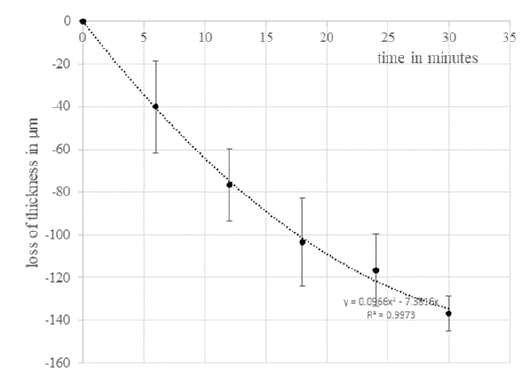
Figure 4: EVEIT corneas in the deficiency medium culture show a
clear swelling of the corneal stroma. The average thickness at the
beginning of the experiment was 760 μm (±58 μm). The change in
corneal thickness was -138 μm (±16 μm) over 30 minutes at 20°C
room temperature and 60% humidity.
Discussion
In the following we want to distinguish between swollen and healthy corneas in terms of perspiratio insensibilis under culture conditions of the EVEIT. Furthermore, a clinical example is made based on physical principles.
Volume Loss
As described above, we observe a difference in the amount of water evaporating from healthy and swollen corneas. One possible explanation for the high evaporation rate in swollen corneas is a less stable epithelium with increased permeability to water. This is suggested by the work of Kinoshita et al. 1995 [14] who found a 4-fold higher fluorescein permeability of the epithelium in patients with corneal stromal edema and an almost 100-fold higher permeability of the epithelium in patients with epithelial edema. Another possible explanation of this difference is the reduced colloidal binding of excess water in the anterior stroma resulting in a facilitated and higher transfer of water towards the epithelium. At the same time, increased permeability of the epithelium results in a water shift with high water loss in the anterior stroma of the cornea (Baudouin et al. 2013) [15].
All these differences in deswelling readily explain the typical improvement in vision and decrease in corneal edema in Fuchs' dystrophy of the cornea over the day. Furthermore, this explains the phenomena of anterior stromal damage in Dry Eye Syndrome (DES) (Messmer et al. 2015) [16]. When looking at the change in corneal thickness versus temperature change, the graph shown in Figure 5 is obtained for healthy versus edematous corneas. There is a significant difference between the two curves, with a swollen cornea losing twice the thickness of a non-swollen cornea at a temperature change of 6°C and almost three times the thickness of a non-swollen cornea at 7°C. This also indicates a significantly higher change in corneal thickness. This also indicates a higher susceptibility of the edematous corneas to evaporation. Furthermore, the temperature drops are steeper due to the increased evaporation. This can be impressively demonstrated in this system of a uniform culture chamber, under stable ambient conditions with defined humidity. With the standard enthalpy of water, this volume loss can be converted into an amount of energy. To evaporate a volume of 0.015 ml requires an energy of 0.015 ml * 0.832 *10 -3 mol at a standard enthalpy (25°C, isobaric) of 43.99 kJ / mol H2O and at a molecular weight of water of 18.01528 g/mol. Accordingly, an energy of 36.6 joules is needed to evaporate this amount of water. In the healthy cornea, this is only 14.11 joules. Conversely, this observation explains the stronger cooling of the edematous cornea.
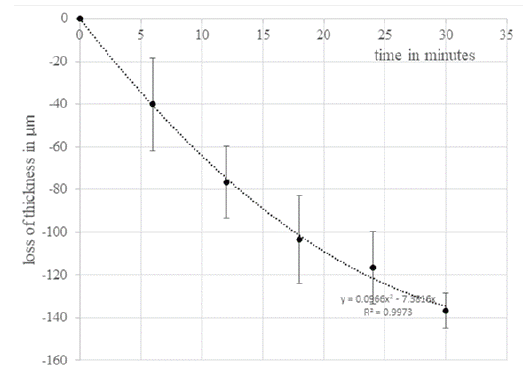
Figure 5: The thickness changes of the healthy cornea are approximately
linear in relation to the temperature change, whereas the
thickness change of the swollen corneas shows hyperbolic dynamics.
Using 3rd degree polynomials, the corneal thicknesses can be mapped against temperature with a good correlation (dashed lines).
Transferred to the patient with DES or Fuchs’s endothelial dystrophy, this results in an earlier trigger for blinking (Schrage et al. 1997) [17] due to the faster cooling of the cornea. This leads to the typical premature eyelid closure in DES (Ding et al. 2021 and Bron 2021) [18,19]. The increased energy required for vaporization of water also explains the typical overheating and reddening of the eyes in DES, as this more than double amount of energy cannot be provided by aqueous humour activity alone. A combination of premature eyelid closure and hyperemia brings in enough blood to maintain the temperature of the cornea.
Clinical Observations
Overall, these results explain the slow increase in swelling of the cornea in the absence of perspiratio insensibilis when the eyelids are closed, e.g., during night sleep, which decreases again during the waking phase over the course of the day. By this there is improved corneal clarity and thus better vision in patients with Fuchs' dystrophy after a distinct time with eye open. This mechanism only works if the endothelium and the perspiratio insensibilis remove more water from the cornea during the daythan flows into the corneal stroma during the night.
With this basic work on corneal physiology, we can physically explain some effects being observed in DES. Another example of perspiratio insensibilis is visible within the clearing of corneal transplants during à chaud keratoplasty with swollen grafts from the European system grafts, which is frequently observed. Furthermore, the effects of excessive water loss from the superficial corneal tissue in dry eye can also be explained. Allevaporation of water leads to a thinning remnant of the salts staying in place with a hyperosmolar load of the epithelia (Cui et al. 2014) [20]. By that an increased salt concentration in the superficial stroma and tears results. This could explain the tear film osmolality of up to 519 mOsmol/kg in DES (Gilbard et al. 1979) [21]. The amount of water evaporated in this test alone (approx. 10% of the honing skin volume) is sufficient to increase the osmolality of 320 mOsmol/kg by 10% to approx. 350-360 mOsmol/kg. This in turn causes stress on the epithelial barrier and its tight junctions and thus changes the epithelial integrity of the. This stress results in permeability leaks of epithelium and might be one trigger of the inflammatory mechanisms of DES.
The marketing departments of various manufacturers of artificial tears propagate hypo-, iso- or hyper-osmolar or osmo- buffers as tear substitutes, depending on the product. Because of our results, a sequence from hyperosmolar to iso- and then to hypo-osmolar seems to be optimal for patients with a DES, and exactly the opposite with corneal edema. This approach probably protects the epithelial barrier of the corneal epithelium and thus supports the improvement of surface permeability as a driving factor of what is happening to the cornea. Notes on the osmolar compositions of the eye drops can be found e.g., in Dutescu et al. (2015) [13].
Finally, our experiments with corneal cooling to 9°C (Figure 3 t>30 minutes) and an increase in corneal thickness confirm the old clinical work of Redbrake et al (1997) [22] and Champman- Smith (1977) [23].
Funding
The research was funded by a limited grant for Ex vivo Eye irritation research of ACTO e.V.
Statements on Ethics Issues
Approval by an ethics committee is not required for the experiments on eyes of animals for slaughter in this case.
Declaration of Pecuniary Interest
The authors have no relevant financial or non-financial interests to disclose.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed mainly by Thomas Schrage with the help of Claudia Panfiland Marc Urbach. The manuscript was written by Thomas Schrage and all authors contributed to the manuscript. All authors read and approved the final manuscript.
References
- Kompa S, Schareck B, Tympner J, Wüstemeyer H, Schrage NF. Comparison of emergency eye-wash products in burned porcine eyes. Graefes Arch Clin Exp Ophthalmol. 2002; 240: 308-13.
- Ehlers N. The fibrillary texture and the hydration of the cornea. Acta Ophthalmol (Copenh). 1966; 44: 620-30.
- Ehlers N. Mechanical factors in the maintenance of normal corneal deturgescence. Acta Ophthalmol (Copenh). 1967; 45: 658-72.
- Kuerten D, Plange N, Koch EC, Koutsonas A, Walter P, Fuest M. Central corneal thickness determination in corneal edema using ultrasound pachymetry, a Scheimpflug camera, and anterior segment OCT. Graefes Arch Clin Exp Ophthalmol. 2015; 253: 1105-9.
- Ehlers N, Hjortdal J. Corneal thickness: measurement and implications. Exp Eye Res. 2004; 78: 543-8.
- Ytteborg J, Dohlman CH. Corneal edema and intraocular pressure. II. Clinical results. Arch Ophthalmol. 1965; 74: 477-84.
- Lindstrom RL. Advances in corneal preservation. Trans Am Ophthalmol Soc. 1990; 88: 555-648.
- Reim M, Hesse R, Pietruschka G. Uber den Stoffwechsel von Organkulturen der Cornea in TC 199 mitZusatz von Dextran 500 oder HAES 450 [The metabolism of organ cultures of cornea in TC 199 with added dextran 500 or hydroxyethyl starch 450]. Clin Monbl Ophthalmology. 1990; 196: 76-80.
- Adamis AP, Filatov V, Tripathi BJ, Tripathi RC. Fuchs’ endothelial dystrophy of the cornea. Surv Ophthalmol. 1993; 38: 149-68.
- Pinheiro R, Panfil C, Schrage N, Dutescu RM. Comparison of the lubricant eyedrops Optive®, Vismed Multi®, and Cationorm® on the corneal healing process in an ex vivo model. Eur J Ophthalmol. 2015; 25: 379-84.
- Amboss.com. 07.05.2021
- Frentz M, Goss M, Reim M, Schrage NF. Repeated exposure to benzalkonium chloride in the Ex Vivo Eye Irritation Test (EVEIT): observation of isolated corneal damage and healing. Altern Lab Anim. 2008; 36: 25-32.
- Dutescu RM, Panfil C, Schrage N. Osmolarity of prevalent eye drops, side effects, and therapeutic approaches. Cornea. 2015; 34: 560-6.
- Yokoi N, Kinoshita S. Clinical evaluation of corneal epithelial barrier function with the slit-lamp fluorophotometer. Cornea. 1995; 14: 485-9.
- Baudouin C, Aragona P, Messmer EM, Tomlinson A, Calonge M, et al. Role of hyperosmolarity in the pathogenesis and management of dry eye disease: proceedings of the OCEAN group meeting. Ocul Surf. 2013; 11: 246-58.
- Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015; 112: 71-81.
- Schrage NF, Flick S, von Fischern T, Reim M, Wenzel M. Temperature changes of the cornea by applying an eye bandage. Ophthalmologe. 1997; 94: 492-5.
- Ding JE, Kim YH, Yi SM, Graham AD, Li W, Lin MC. Ocular surface cooling rate associated with tear film characteristics and the maximum interblink period. Sci Rep. 2021; 11: 15030.
- Bron AJ, Willshire C. Tear Osmolarity in the Diagnosis of Systemic Dehydration and Dry Eye Disease. Diagnostics (Basel). 2021; 11: 387.
- Cui X, Hong J, Wang F, Deng SX, Yang Y, Zhu X, Wu D, Zhao Y, Xu J. Assessment of corneal epithelial thickness in dry eye patients. Optom Vis Sci. 2014; 91: 1446-54.
- Gilbard JP, Farris RL. Tear osmolarity and ocular surface disease in keratoconjunctivitis sicca. Arch. Ophthalmol. 1979; 97: 1642- 1646.
- Redbrake C, Salla S, Frantz A, Reim M. Studies on the energy metabolism of the human cornea in various culture systems. Klin Monbl Augenheilkd. 1997; 210: 213-8.
- Chapman-Smith JS. A further examination of short-term corneal storage. Invest Ophthalmol Vis Sci. 1977; 16: 556-9.