
Review Article
J Ophthalmol & Vis Sci. 2023; 8(1): 1075
Role of Topography in Cataract Surgery Planning: Narrative Review
Bonacci E¹, Anastasi M¹*, Pagnacco C¹, Marchini G² and Pedrotti E³
¹Deparment of Neuroscience Biomedicine and Movements, University Hospital of Verona, Italy
²Chairman and Full Professor of Ophthalmology, Deparment of neuroscience biomedicine and moovments, University Hospital of Verona, Italy
³Associate Professor of Ophthalmology, Deparment of neuroscience biomedicine and moovments, University Hospital of Verona, Italy
*Corresponding author: Marco Anastasi University Hospital of Verona, Verona, Italy
Received: January 04, 2023; Accepted: February 11, 2023; Published: February 18, 2023
Abstract
Corneal topography is a useful investigation in the preoperative cataract surgery planning, becoming mandatory when full refractive outcome is required. Evaluation of corneal power, corneal astigmatism and its management are pivotal to both exclude ectasia, assess the corneal shape and for patient selection. This paper reviews the principles of successive generations of topographers and illustrates several normal and abnormal corneal topographies giving useful indications for refractive cataract surgery.
Keywords: Corneal Topography; Corneal Astigmatism; Preoperative Evaluation; Cataract Surgery; Refractive Cataract Surgery
Background
The cornea is the most important positive lens to be evaluated during cataract surgery planning and the acquisition of its optical measurements, needed to optimize Intraocular Lens (IOL) selection, still betrays the ophthalmologist. Main factors causing errors were the effective lens position, the ocular axial length and the corneal measurements. However, while advances in optical biometrics have increased the accuracy of axial length measurements reaching errors smaller than 0.05 Diopters (D), the effective lens position cannot currently be measured prior to surgery, despite improvements in its estimation have been achieved thanks to the inclusion of new parameters in the IOL calculation formulas [1]. What ophthalmologists can do to increase the accuracy in the IOL calculation and consequently the refractive outcome is to obtain more accurate and complete measurements of corneal power [2].
However, currently the topography is not included in the official standard preoperative workup for cataract surgery [3] leading to the potential misdiagnosis of several corneal diseases (irregular corneal shape, not advanced keratoconus, pellucid marginal degeneration, etc.) which are generally not identified with keratometry, biometry and slit lamp (standard preoperative tests). Only by topography the shape of corneas underwent to previous surgery (refractive, transplants or other incisions) is properly detected. Failure in these evaluations and the following errors in the corneal power calculation led to even considerable refractive errors [4].
Furthermore, the cornea acts for two thirds of the eye's total focusing power, so very small changes in the corneal shape induce an amplified effect on the light deflection and so on the refraction. Since that, the incisions performed during cataract extraction can modify the corneal refraction. To take it in consideration when performing refractive pseudophakic surgery is mandatory. Corneal topography assessments can be used to minimize the negative results of these incisions and even use their effects to advantage [5].
Last but not least, the increasing use of Advanced Technology Intraocular Lenses (ATIOLs), stressed the need for careful preoperative corneal evaluation for both IOL determination and a patient's selection [5].
Objectives
The aim of this review is to focus on the role of the topography in the planning of cataract surgery.
Methods
The systematic review was performed using databases: PubMed (National Center for Biotechnology Information; https://www.ncbi.nlm.nih.gov/pubmed,), and Cochrane library (latest search conducted on September 30, 2022). Using meSH words linked by AND “Cornea” “Topography” “Cataract” “Refractive”; The Pubmed and Cochrane library searches yielded 1432 + 42 records. Filter between 2010 and 2022 (707 + 21) including clinical trial, meta-Analysis, randomized controlled trials, review and systematic review was applied and finally the search yielded 85+ 21 records.
Pubmed results, already obtained from the previous database, were excluded from the Cochraine research. Case series, pilot studies and studies with inadequate sample sizes were excluded. Articles were selected for review on the basis of content and referenced articles. Pertinent data and information were integrated into this review.
Review
Current Corneal Topography Technologies
Placido disc has stood the test of time and the current placido based topographers work on the same principle of assessing the reflection of a concentric set of black and white rings from the convex anterior surface of the cornea. Indeed, the first- generation of corneal topographers are devices that project a concentric light circles system (the Placido disk) onto the corneal surface, measure their angle of reflection and thus calculate the corneal curvature at various points, providing information on the corneal shape. Second-generation of corneal topographers, Orbscan® type (Bausch and Lomb, USA), assesses the corneal elevation through optical sections obtained by combining Placido disc and slit scanning technologies, thus being able to characterize the posterior face of the cornea. Corneal tomographers are the third-generation devices, their technology allowing digital reconstruction of the structures of the anterior segment, without using a curvature system. The term “tomography” is also derived from the Greek words “tomos” (section) and “graphein” (to write). The technology of corneal tomographers uses a Scheimpflug type rotating camera that allows the analysis of both anterior and posterior faces of the cornea, by direct measurements, not only by mathematical assessments as in the case of topographers. In contrast, Scheimpflug technology provides less information on possible distortion of the anterior corneal face compared to the Placido disc. Modern capture and analysis systems that combine the Scheimpflug rotating camera, the Placido disk and the slit scanning, such as the Galilei® (Ziemer, Switzerland) and Sirius® (Schwind Eye-Tech-Solutions, Germany), were created to combine the advantages of topographers and tomographers [6,7].
Corneal Refractive Power
Corneal power assessment during preoperative workup for refractive cataract surgery is mandatory for IOL power calculation because it affects the deviation of light rays on the retina [8].
The standard corneal power display is sim K (simulated keratometries), which in most devices is calculated as the mean between the steeper and flatter corneal curvature values in 3-4 mm anular zone instead, in Scheimpflug devices the average corneal power is calculated using data from all meridians [9].
Causes of intra- and inter-device corneal power measurement variability, including tear film abnormalities, corneal surface irregularities, technician error and device variability could reduce the accuracy of IOL calculations. Koch in a recent work studied the repeatability of 3 measurements made by four device/software outputs were tested: Humphrey Atlas (Carl Zeiss) SimK, Galilei Dual Scheimpflug Analyzer Sim K (Ziemer, Port, Switzerland), IOL Master 500 (Carl Zeiss), and manual keratometry (Bausch & Lomb, Inc, Rochester, New York, USA). He found that intra-device standard deviations and coefficients of variation were low, suggesting acceptable clinical repeatability, while the inter-device differences were greater, albeit generally clinically acceptable, with 95% limits of agreement for mean corneal power ranging from 0.25 D for Galilei-IOL Master to over 0.5 D for Atlas/manual keratometer and Atlas/Galilei. This magnitude of variability could certainly reduce the accuracy of IOL calculations. The commonly employed solution to mitigate errors from inter-device differences is to optimize lens constants based on corneal data from one device. However, one advantage of using more than one device is detection of erroneous measurements [2].
For this reason, in case of healthy corneas and with regular astigmatism, the small variability of the keratometry readings gives an accuracy of IOL power within the 0.5 D step. In the same work, Abulafia reports the greater calculation precision in healthy corneas with devices that measure both keratometry and axial length, as the algorithms within the device are calibrated using those [10,11].
On the other hand, in eye showing irregular corneal astigmatism, visual results may not be as good as expected, and corneal topography is crucial, especially because routine examinations with a slit lamp and keratometer are not enough to evaluate it [12]. In 2015 Loh J. showed a higher IOL power prediction using corneal topography than keratometry in measuring the corneal curvature in case of irregular astigmatism [5]. However, surgeons meet the difficulty of choose which data to use for the IOL power calculations: Keratometric equivalent at the 3 mm zone (average of the steepest and flattest meridians) Average curvature of the 3 mm ring, Average curvature of the 4 mm ring, Mean central corneal power, Centrally weighted mean corneal power, Mean pupillary power. ecc. As a general guideline, measurements that use a larger number of data points from the area closest to the central cornea are more useful.
Although the anterior corneal surface takes the most refractive effect, for a correct evaluation of the corneal power it is also mandatory to consider its posterior surface [10,13,14].
Corneal Astigmatism
Astigmatism can be defined as the refractive error in which no focal point is achievable after the light deflection through the cornea, due to the unequal refraction of light in the different meridians. It can result from asymmetry or decentralization of the optical surfaces of the eye or irregularities in the refractive index.
Its prevalence was estimated to be 86.6%. The 40% of these refers to corneal astigmatism higher than 1.0 Diopters (D) and 20% higher than 1.5 D [15].
To evaluate pre-existing corneal astigmatism is mandatory to obtain higher refractive outcome after cataract surgery, and the topography is able to evaluate both regularity and the magnitude of corneal astigmatism [8].
In regular astigmatism, corneal topography displays a symmetrical bow-tie pattern with the principal meridians (of greatest and least powers) being located 90 degrees apart. In the with-the-rule astigmatism, which is the more common form of regular astigmatism, the steepest radius and the bow-tie pattern are located in the vertical meridian. Less frequent types of regular astigmatism are the against-the-rule astigmatism, when the bow- tie pattern and the steepest meridian are located horizontally and the oblique astigmatism, when both the steepest meridian and the bow-tie pattern are diagonally placed.
Irregular astigmatism occurs when two halves of the cornea are consistently different (superior versus inferior or nasal versus temporal). The two sides of the bow-tie differ in magnitude (asymmetrical bow-tie) or are not orthogonally aligned to each other (skew of steepest radial axes) or both [16]. Irregular corneal astigmatism can occur both in a healthy cornea and in diseased one (corneal dystrophy, keratoconus, leukomas, scars, prior keratoplasty or refractive surgery [7].
Furthermore, posterior astigmatism evaluation has to be considered. Ignoring posterior corneal astigmatism may yield incorrect estimation of total corneal astigmatism leading overestimation of with-the-rule astigmatism by 0.5 D and underestimation of against-the-rule astigmatism by 0.3 D. Other studies have shown that ignoring posterior astigmatism may lead to axis errors of 7.4° ± 10.3° [14].
Knowledge of the magnitude, location and regularity of pre-existing astigmatism is crucial during the planning of cataract surgery.
Vector analysis can be used to calculate the surgical induced astigmatism, which needs to be added to the existing astigmatism in order to produce the desired result [8].
Regular Astigmatism
The pre-existing regular corneal astigmatism could be managed in different ways to aim satisfactory post-operative results:
by using the appropriate placement and construction of the incision, by centering the incision on the steep meridian and using a wound construction-closure combination that will produce the required astigmatic decay;
By Corneal Relaxing Incisions (CRIs) as Astigmatic Keratotomy (AK), peripheral limbal relaxing incisions (LRIs) and opposite clear corneal incision (OCCI) [17].
Thirdly, a toric intraocular lens can be implanted [18].
Planning the incisions
A superior main incision is recommended for with-the-rule astigmatism having a steep axis >1.5 D. Temporal incision is recommended for: against-the-rule astigmatism <0.75 D or negligible astigmatism, an additional nasal incision is recommended for >0.75 D [17].
AKs induce flattening of the steeper meridian and stiffening of the flatter meridian (coupling effect), with 1:1 ratio. AKs reduce astigmatism without major changes in the spherical equivalent. They can be performed before or during cataract surgery, either manually or by femto second laser. LRIs are often performed in conjunction with cataract surgery in order to treat corneal astigmatism from 0.5 to 1.0 D. They can be used also for higher degrees (up to 2.0 D) but they tend to be less accurate. LRI can cause a slight farsightedness shift of approximately 0.20 D, which should be considered when selecting the IOL power [17].
OCCI consists in performing an identical, penetrating clear corneal incision opposite to the main one, to enhance the flattening effect in reducing the pre-existing corneal astigmatism. The OCCI can reduce pre-existing corneal astigmatism up to 2.0 D [17].
Toric IOL
Approximately 30% of patients waiting for cataract surgery have corneal astigmatism higher than 1.0 D and 22% higher or equal to 1.5 D [19].
Toric IOL surgery requires an accurate analysis of the type of corneal pre-existent astigmatism both anterior and posterior, and choosing the most suitable technique, depending on the type of astigmatism and its magnitude [6]. Mono- and multifocal toric implants are used to treat high degrees of astigmatism, up to 12 D, but risk of rotation or misalignment exist. Total correction of corneal astigmatism is imperative for optimal results in multifocal toric implants6and a carefully study of the corneal topography is mandatory. For astigmatisms between 0.50 and 0.75 D, it is possible to perform both a corneal incision on steeper meridian and a toric implant. For astigmatism higher than 0.75 D, a toric implant is recommended avoiding corneal incision techniques for its unpredictable result. Higher outcomes after toric implants have been reported in patients with cataract and regular corneal astigmatism [20,21] because toric IOLs only provide a symmetrical correction. The same high results cannot be usually expected in patients with corneal irregular astigmatism [22] for which the toric IOLs goal is to improve the visual comfort decreasing spectacle dependence. However, several recent works show the efficacy of toric implants in the surgical management of irregular astigmatism in keratoconus [23], in pellucid marginal degeneration [24] or after corneal transplantation [25] (Figure 1). Yi Gao and co-workers found that patients having irregular astigmatism with a regular central component can be considered for toric IOL implantation [26].
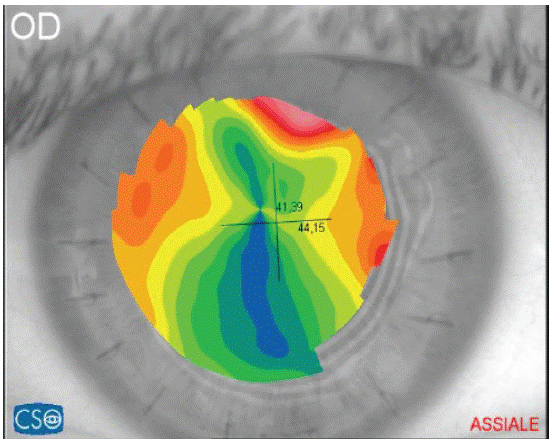
Figure 1: Corneal Regular Astigmatism post PKP. Patient suitable for Toric-IOL surgery after sutures removal.
However, it is important to be cautious when selecting a toric lens for the management of irregular astigmatism because surgery meets a several troubles: biometric accuracy, astigmatism management (corneal topography plays a crucial role in both) and IOL calculation.
Toric IOL calculators have also become more sophisticated to predict residual postoperative astigmatism. Generally, there are 2 approaches for toric IOL calculations. The fi rst is to use mathematical models (e.g., Barrett Toric Calculator, Abulafi a-Koch) that estimate the predicted postoperative residual refractive astigmatism based on anterior corneal measurements, and the second is the use of total corneal astigmatism measurements (Scheimpfl ug imaging with Panacea Toric Calculator, ray tracing software such as PhacoOptics, Aarhus Nord, Denmark). Studies comparing the 2 different approaches found that the direct measurement approach was not superior to the estimated mathematical approaches. However, subgroup analysis suggests that all calculators still tend to overcorrect with-the-rule astigmatism and under correct against the-rule astigmatism [27].
Irregular Astigmatism
In case of irregular astigmatism, it would be advisable to diagnose the cause and treat it before performing cataract surgery, because it induces refractive errors and high-order aberrations that would lead to a significant deterioration in the quality of vision [28]. Anterior corneal dystrophies or sub-epithelial leukomas or scars, could be subtle and easily overlooked during routine examination and it can be identified by irregular astigmatism on topography (Figure 2). Consequences of the missed diagnosis can lead to unwanted refractive results and unhappy patients for inaccurate keratometry readings [28]. Furthermore, the topography can assess the severity and stability of the condition. If the lesion does not act the visual axis and no progression is viewed, a standard IOL with an asymmetrical corneal incision on the steeper side could be performed to manage asymmetric astigmatism. Moreover, it can be recommended to avoid ATIOLs. If the lesion leads to significant regular astigmatism, the patient may benefit from a toric IOL. However, if the lesion acts the visual axis or causes irregular astigmatism, its treatment by phototherapeutic keratectomy before cataract surgery could lead to either resolving the astigmatism or leaving a mild and regular one [29] (Figure 3).
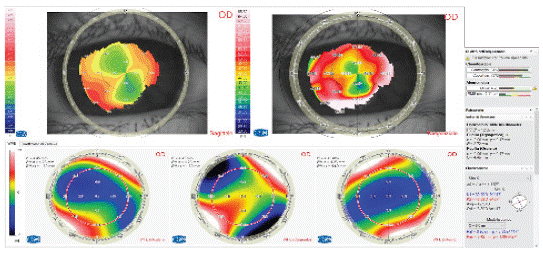
Figure 2: Recurrent Keratoconus detectable by tangential map and corneal wave front.
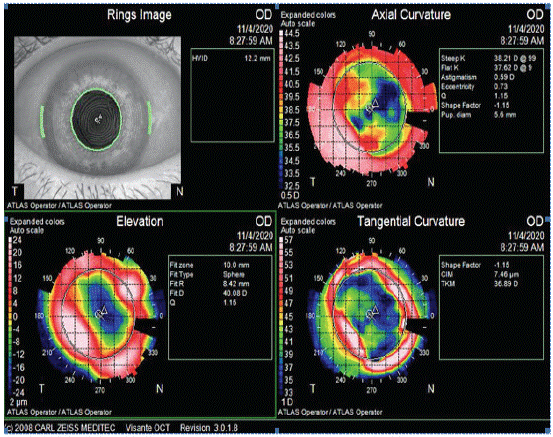
Figure 3: Irregular Astigmatism, due to corneal leukoma, secondary to infection. Patient is waiting for PTK before cataract surgery.
After refractive surgery
The IOL power calculation after corneal refractive surgery represents one of the most challenges for the cataract surgeon due to the high expectations of visual outcomes and to the difficulties of IOL power prediction. IOL power calculation can be wrong for three reasons: keratometry index, curvature radius and effective lens position.
Keratometry index: this index used in the cornea power formula, assumes a fixed ratio between the anterior and posterior corneal curvature. This ratio changes after refractive corneal surgery (laser-assisted in situ keratomileusis-LASIK or photorefractive keratectomy- PRK), because the anterior corneal curvature changes but not the posterior one, leading to an overestimation of corneal power after myopic surgery and an underestimation after hypermetropic surgery. For corneas that have undergone radial keratotomy, the posterior corneal curvatures change, but in unpredictable ways [3,30]. The Scheimpflug camera devices are able to measure the total corneal power evaluating both anterior and posterior corneal surfaces, so solves the error due to the keratometry index. The total corneal power is measured by ray-tracing and in the not operated eye is 0.7 D lower than simK, so using it in classic IOL formulas is not possible, unless the constant is optimized. This value is Total Corneal Power in Galilei device (Ziemer); Total Corneal Refractive Power or True Net Power in Pentacam device (Oculus); Mean Pupillary Power in Sirius device (CSO) [31].
Curvature radius: topography measures the anterior corneal curvature not on the visual axis, but in the central annular zone between 2.4 and 3.3 mm. This corneal curvature is assumed equal at the central one, considering the anterior corneal surface is a sphere without difference between central and paracentral area. This assumption is valid for the not operated eye, but not after refractive corneal surgery, especially in surgery involving a small optical zone or large attempted correction or both, as well as those with low postoperative keratometry readings. These errors are negligible with myopic treatments having an optical zone of 6-6.5 mm [32].
Predicted lens position: many IOL calculation formulas use corneal power values in their calculations to predict the Effective Lens Position (ELP). Following LASIK, PRK, or RK, corneal power is altered, and the predicted ELP would be misleading if the postoperative corneal power is used. To avoid the ELP-related IOL prediction error, the double-K method proposed by Aramberriet al. could be used [33].
Ectasia
Detecting an ectatic corneal disease (keratoconus, pellucid marginal degeneration, ectasia post corneal refractive surgery, recurrence keratoconus [34], apnea syndrome [35] etc.) is crucial before the cataract surgery and it is hardly to diagnose by slit lampe especially in their early stage. Topography is essential to diagnose corneal ectasia, to assess its stability, to evaluate Higher-Order Aberrations (HOAs) and to avoid IOL power calculation errors [36]. For these reasons, initially keratometricdatas were used to differentiate healthy eyes from ectatic eyes [37] demonstrating 0.80% sensitivity and 0.70% specificity for keratometric cutoff [38], however, this parameter is poor for the detection of subclinical disease [39]. So, several topographic parameters have been developed as a predictor of ectasia [40] as the I–S index, which represents the amount of steepening of the inferior cornea compared with that of the superior cornea; the SAI, surface asymmetry index; SRI, surface regularity index; CIM, corneal irregularity measurement; MTK, mean toric keratometry; SRAX, skew of steepest radial axis; CSI, center surround index; DSI, different sector index; and OSI, opposite sector index, the keratoconus prediction index, to classify subjects according to the shape of the anterior corneal surface [41]; the keratoconus percentage index (KISA%) value is calculated from a combination of 4 video keratographic parameters having an accuracy of 99.6% [42], Keratoconus Index (KCI) able to distinguish between keratoconus developed in the central or the peripheral regions, Keratoconus Prediction Index (KPI) calculated by a combination of 8 topographic indices and the ocular residual astigmatism (ORA) (sensitivity 82%, specificity 92%) showing sensitivity of 68 % and a specificity of 99 % [39,43].
Sources of error in ectatic corneas are IOL power calculation formulas and HOAs (due tocorneal irregular astigmatism and shape) as described above. The visual restore for ectatic corneas requires a specific flowchart (when it is possible) addressing three concerns: halting the ectatic process, improving corneal shape and minimizing the residual refractive error. In case of ectasia progression, cross-linking performed at least three months before the cataract surgery can halt the disease progression. Furthermore, toric IOL implantation cannot guarantee high results in patients with corneal irregular astigmatism secondary to ectasia and LRI should to be avoided because they can worsen the ectasia. In this case using corneal refractive power (Mean central corneal power, centrally weighted mean corneal power, Mean pupillary power etc) instead of sim-k is recommended [42].
The intrastromal corneal ring segments are indicated in these patients because they can improve the corneal shape regularizing the astigmatism, reducing the refractive error amount thus leading to a better result with toric IOL. Despite all the difficulties met by the surgeon during cataract planning in patients with ectasia, it is well known that these patients generally have a better tolerance to defocus than healthy patients so, some residual refractive errors after IOL implantation can be better tolerated [44] (Figure 4a-b).
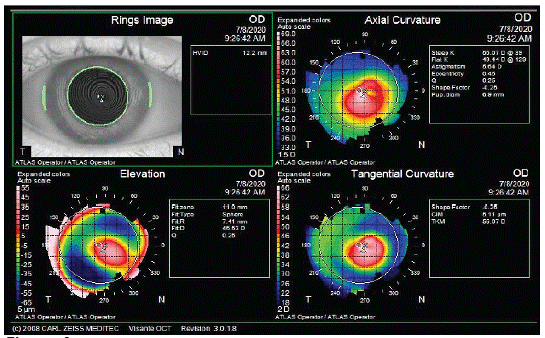
Figure 4a:
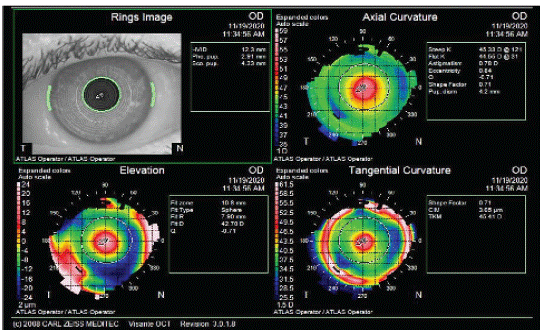
Figure 4b: Keratoconus patient treated by Intracorneal segment Rings (INTACS) before cataract surgery to manage his irregular astigmatism.
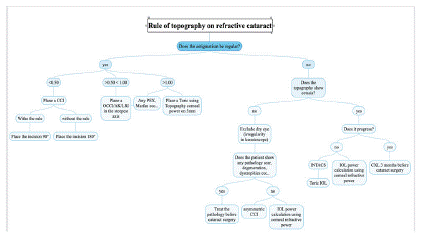
Figure 5: Management of corneal astigmatism in refractive cataract surgery.
Although HOA are a small part of the refractive errors in normal eyes, they can negatively influence the quality of vision, especially in mesopic contrast sensitivity. Since the cornea is the most powerful refractive element of the eye, corneal aberrometry represents the 70-80% of the total ones of the eye. So, corneal wave front evaluation is pivotal in the preoperative path of cataract surgery, especially to evaluate the suitability for the advanced technology lens implant. Refractive outcomes in cataract surgery, indeed, include not only the visual acuity restoration but also higher quality of vision. The cornea typically induces positive spherical aberration, which in young people is offset by the negative spherical aberration of the lens. Getting older, spherical aber ation of the lens shifts to positive values which results in a worsening of optical quality [45]. Spherical monofocal IOLs can introduce a new positive spherical aberration, adding approximately 0.08 μm (over a 4 mm pupil) to the pre-existing corneal ones. Aspheric IOLs, instead, generate negative spherical aberration, leading to a smaller amount of postoperative spherical aberration as compared to spherical IOLs, increasing visual quality outcomes [45].
While, the finding of a negative spherical corneal aberration should discourage the choice of anegative aspherical intraocular lens. Corneal refractive surgery leads to modification in the anterior surface asphericity. A positive spherical aberration is induced in cornea undergoing myopia treatments and aspheric IOLs are recommended, whereas a negative spherical aberration is induced in hyperopic treatments so the best choice is the classic spherical IOL or ones with free aspherical aberrations [45].
Unfortunately, the other corneal HOAs (coma, trefoil, pentafoil, coma II and III etc) cannot be corrected, and they negatively affect the quality of vision so, their presence must be evaluated especially when considering an advanced implant. Several studies found that values up to 0.3 μm cut off of HOA, based on pupil diameter of 3-4 mm, produce 0.5 D of defocus which theoretically corresponds to the root mean square (RMS, μm) value of 0.29 μm. Values higher than 0.3 μm cut off of HOA indicated that implantation of advanced technologies IOL should be avoided for low satisfaction outcomes and intolerable dysphotopsia furthermore should prompt the surgeon to investigate for corneal pathologies causing irregular astigmatism (KCN, scars, dystrophies etc) [46,47].
Given the essential information that the topography provides to the cataract surgeons, his absence from the official standard preoperative workup for cataract is to be considered and a serious gap.
Funding Sources
No funding was received for this study.
Authors’ Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Emilio Pedrotti and Giorgio Marchini contributed equally to this study.
References
- Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008; 34: 368-76.
- Koch DD. The Enigmatic Cornea and Intraocular Lens Calculations: The LXXIII Edward Jackson Memorial Lecture. Am J Ophthalmol. 2016; 171: xv-xxx.
- American Academy of Ophthalmology Cataract and Anterior Segment Panel, Preferred Practice Pattern Guidelines, Cataract in the Adult Eye. San Francisco, CA: American Academy of Ophthalmology, 2011.
- Loh J. Importance of Performing Corneal Topography before Cataract Surgery. US Ophthalmic Review. 2015; 08: 92.
- Hayashi K, Yoshida M, Hirata A, Yoshimura K. Changes in shape and astigmatism of total, anterior, and posterior cornea after long versus short clear corneal incision cataract surgery. J Cataract Refract Surg. 2018; 44: 39-49.
- Doctor K, Vunnava KP, Shroff R, Kaweri L, Lalgudi VG, Gupta K, Kundu G. Simplifying and understanding various topographic indices for keratoconus using Scheimpflug based topographers. Indian J Ophthalmol. 2020; 68: 2732-2743.
- Moshirfar M, Motlagh MN, Murri MS, Momeni-Moghaddam H, Ronquillo YC, Hoopes PC. Galilei Corneal Tomography for Screening of Refractive Surgery Candidates: A Review of the Literature, Part II. Med Hypothesis Discov Innov Ophthalmol. 2019; 8: 204-218.
- Fung MW. Corneal topography and imaging. Medscape Ophthalmology 2014; updated 2016.
- Tonn B, Klaproth OK, Kohnen T. Anterior surface–based keratometry compared with Scheimpflug tomography–based total corneal astigmatism. Invest Ophthalmol Vis Sci. 2015; 56: 291–298.
- Abulafia A, Barrett GD, Kleinmann G, Ofir S, Levy A, Marcovich AL, Michaeli A, Koch DD, Wang L, Assia EI. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015; 41: 936-44.
- Brandsdorfer A, Kang JJ. Improving accuracy for intraocular lens selection in cataract surgery. Curr Opin Ophthalmol. 2018; 29: 323-327.
- Gao Y, Ye Z, Chen W, Li J, Yan X, Li Z. Management of Cataract in Patients with Irregular Astigmatism with Regular Central Component by Phacoemulsification Combined with Toric Intraocular Lens Implantation. J Ophthalmol. 2020; 2020: 3520856.
- Fityo S, Bühren J, Shajari M, Kohnen T. Keratometry versus total corneal refractive power: Analysis of measurement repeatability with 5 different devices in normal eyes with low astigmatism. J Cataract Refract Surg. 2016; 42: 569-76.
- Koch DD, Jenkins RB, Weikert MP, Yeu E, Wang L. Correcting astigmatism with toric intraocular lenses: Effect of posterior corneal astigmatism. J Cataract Refract Surg. 2013; 39: 1803–1809.
- Mohammadi M, Naderan M, Pahlevani R, Jahanrad A. Prevalence of corneal astigmatism before cataract surgery. Int Ophthalmol. 2016; 36: 807-e817.
- Goto S, Maeda N. Corneal topography for intraocular lens selection in refractive cataract surgery. Ophthalmology. 2020; 128: e142-e152.
- XY Sun, YZ Li, T Qian. Correction of corneal astigmatism by topography-guided incision in cataract surgery. March 2010 International Journal of Ophthalmology. 10: 462-465
- Nikose AS, Saha D, Laddha PM, Patil M. Surgically induced astigmatism after phacoemulsification by temporal clear corneal and superior clear corneal approach: a comparison. Clin Ophthalmol. 2018; 12: 65-70.
- Lyall DA, Srinivasan S, Ng J, Kerr E. Changes in corneal astigmatism among patients with visually significant cataract. Can J Ophthalmol. 2014; 49: 297-303.
- M Kaur, F Shaikh, R Falera, Titiyal J. Optimizing outcomes with toric intraocular lenses, Indian Journal of Ophthalmology. 2017; 65: 1301–1313.
- L Kessel, J Andresen, B Tendal, D Erngaard, P Flesner, et al. Toric intraocular lenses in the correction of astigmatism during cataract surgery. Ophthalmology. 2016; 123: 275–286.
- Savini G, Næser K. An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Investig Ophthalmol Vis Sci. 2015; 56: 827–835.
- Yahalomi T, Achiron A, Hecht I, Arnon R, Levinger E, Pikkel J, et al. Refractive Outcomes of Non-Toric and Toric Intraocular Lenses in Mild, Moderate and Advanced Keratoconus: A Systematic Review and Meta-Analysis. J Clin Med. 2022; 11: 2456.
- Luck MD. Customized toric intraocular lens implantation for pellucid marginal degeneration and cataract. J Cataract Refract Surg. 2010; 36: 1235-8
- Devebacak A, Degirmenci C, Barut Selver O, Palamar M, Egrilmez S. Correction of high astigmatism with toric intraocular lens in eyes with corneal transplant. Eur J Ophthalmol. 2022: 11206721221123885.
- M. Ilse, VD Bart. Toric intraocular lenses for correction of astigmatism in keratoconus and after corneal surgery. Clinical Ophthalmology. 2016; 10: 1153-1158.
- Ferreira TB, Ribeiro P, Ribeiro FJ, Neill JG. Comparison of methodologies using estimated or measured values of total corneal astigmatism for toric intraocular lens power calculation. J Refract Surg. 2017; 33: 794-800.
- Chuang J, Shih KC, Chan TC, Wan KH, Jhanji V, Tong L. Preoperative optimization of ocular surface disease before cataract surgery. J Cataract Refract Surg. 2017; 43: 1596-1607.
- Donaldson K, Fernández-Vega-Cueto L, Davidson R, Dhaliwal D, Hamilton R, Jackson M, Patterson L, Stonecipher K; ASCRS Refractive–Cataract Surgery Subcommittee. Perioperative assessment for refractive cataract surgery. J Cataract Refract Surg. 2018; 44: 642-653.
- Ganesh S, Patel U, Brar S. Posterior corneal curvature changes following Refractive Small Incision Lenticule Extraction. Clinical Ophthalmology. 2015; 9: 1359-1364.
- Savini G, Bedei A, Barboni P, Ducoli P, Hoffer KJ. Intraocular lens power calculation by ray-tracing after myopic excimer laser surgery. Am J Ophthalmol. 2014; 157: 150-153.e1.
- Savini G, Hoffer KJ. Intraocular lens power calculation in eyes with previous corneal refractive surgery. Eye Vis (Lond). 2018; 5: 18.
- Awwad ST, Kilby A, Bowman RW, Verity SM, Cavanagh HD, et al. The accuracy of the double-K adjustment for third-generation intraocular lens calculation formulas in previous keratorefractive surgery eyes. Eye Contact Lens. 2013; 39: 220-7.
- Pedrotti E, Caldarella G, Fasolo A, Bonacci E, Gennaro N, et al. Topographic and Biomechanical Changes after Application of Corneal Cross-Linking in Recurrent Keratoconus. Int J Environ Res Public Health. 2019; 16: 3872.
- Pedrotti E, Demasi CL, Fasolo A, Bonacci E, Brighenti T, et al. Obstructive Sleep Apnea Assessed by Overnight Polysomnography in Patients With Keratoconus. Cornea. 2018; 37: 470-473.
- Gomes JA, Tan D, Rapuano CJ, Belin MW, Ambrósio Jr R, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015; 34: 359–69.
- Kamiya K, Shimizu K, Igarashi A, Miyake T. Assessment of anterior, posterior, and total central corneal astigmatism in eyes with keratoconus. Am J Ophthalmol. 2015; 160: 851–857.
- Shetty R, Kaweri L, Pahuja N, Nagaraja H, Wadia K, et al. Current review and a simplified "five-point management algorithm" for keratoconus. Indian J Ophthalmol. 2015; 63: 46-53.
- Reddy JC, Rapuano CJ, Cater JR, Suri K, Nagra PK, et al. Comparative evaluation of dual Scheimpflug imaging parameters in kerato-conus, early keratoconus, and normal eyes. J Cataract Refract Surg. 2014; 40: 582–592
- Martínez-Abad A, Piñero DP. New perspectives on the detection and progression of keratoconus. J Cataract Refract Surg. 2017; 43: 1213-1227.
- Cavas-Martínez F, De la Cruz Sanchez E, NietoMartínez J, Fernandez- Canavate FJ, Fernandez-Pacheco DG. Corneal topography in keratoconus: state of the art. Eye Vis. 2016; 3: 5.
- Smadja D. Topographic and tomographic indices for detecting keratoconus and subclinical keratoconus: a systematic review. Int J Keratoconus Ectatic Corneal Dis. 2013; 2: 60–64.
- Ramos-Lopez D, Martínez-Finkelshtein A, Castro-Luna GM, Burguera- Gimenez N, Vega-Estrada A, Pinero D, Alio JL. Screening subclinical keratoconus with Placido-based corneal indices. Optom Vis Sci. 2013; 90: 335–343.
- Fernández-Vega-Cueto L, Romano V, Zaldivar R, Gordillo CH, Aiello F, Madrid-Costa D, Alfonso JF. Surgical Options for the Refractive Correction of Keratoconus: Myth or Reality. J Ophthalmol. 2017; 2017: 7589816.
- Schrecker J, Langenbucher A, Seitz B, Eppig T. First results with a new intraocular lens design for the individual correction of spherical aberration. J Cataract Refract Surg. 2018; 44: 1211-1219.
- Braga-Mele R, Chang D, Dewey S, Foster G, Henderson BA, et al. Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg. 2014; 40: 313–22.
- Hamza I, Aly MG, Hashem KA. Multifocal IOL dissatisfaction in patients with high coma aberrations. Presented at: ASCRS Symposium on Cataract, IOL and Refractive Surgery; March 27, 2011; San Diego, CA.