
Case Series
J Ophthalmol & Vis Sci. 2023; 8(1): 1076.
Vitrectomy for Macular Hole with Macular Foveoschisis in Myopic Tractional Maculopathy PPV for MH-MFS
Rodríguez-Vargas G1,2*, Ortiz-Coto F1,2, Burés-Jelstrup A¹ and Mateo C¹
¹IMO – Instituto de Microcirugía Ocular, Barcelona, Spain
²UCR – Universidad de Costa Rica, San José, Costa Rica
*Corresponding author: Rodríguez-Vargas GGabriel Rodríguez-Vargas, Edificio Dualoft, Apt 29, San José, Costa Rica
Received: January 13, 2023; Accepted: February 16, 2023; Published: February 23, 2023
Abstract
Background: Macular Hole with Macular Foveoschisis (MH-MFS) represents one of the end stages of Myopic Traction Maculopathy (MTM). This study demonstrates the effectiveness of Pars Plana Vitrectomy (PPV) with appropriate Internal Limiting Membrane (ILM) peeling and use of an ILM flap without the conventional use of macular buckle for resolving this condition.
Methods: Twelve highly myopic eyes with MH-MFS underwent PPV in a single institution. Careful fovea sparing Inner Limiting Membrane (ILM) peeling with inverted flap under repeated re-staining with brilliant blue was performed. Best Corrected Visual Acuity (BCVA) and rate of Macular Hole (MH) closure, as measured by Optic Coherence Tomography.
Results: Primary MH closure was obtained in 90.9% of the cases. Best Corrected Visual Acuity (BCVA) improved in 9 patients (75%) from a baseline BCVA of 0.59 ± 0.26 log MAR (20/77) to a post-operatory BCVA of 0.35 ± 0.25 log MAR (20/44) with statistical significance (p=0.004). Foveoschisis resolution occurred as follows: 6 cases within the first month, and 9 cases by the first year. Partial resolution was achieved in 2 cases, while one failed to resolve during follow-up.
Conclusions: PPV with ILM peeling and inverted flap is a safe and effective method for managing MH-MFS. Careful identification of ILM and a thorough understanding of the vitreomacular interface abnormalities is key to surgical success.
Keywords: Myopic Foveoschisis, Macular Hole with Maculoschisis, Myopic Traction Maculopathy
Background
High myopic patients have been described to be at high risk for developing Macular Holes (MH). This entity has been reported to be the most frequent cause for MH formation in patients younger than 50 years [1]. A subtype of MH, called Macular Hole with Macular Foveoschisis (MH-MFS) is characterized by the presence of retinoschisis secondary to a posterior staphyloma in highly myopic patients [2-4].
Myopic Tractional Maculopathy (MTM) is a term coined by Panozzo G. in 2014 to describe the spectrum of tractional foveal changes in highly myopic eyes [5]. These changes are caused by a progressive enlargement and elongation of the sclera, resulting in stretching of the choroidal-retinal pigmented epithelium-retina complex in two directions, both perpendicular and/or tangential to the retina [6]. MH-MFS represents one of the end stages of MTM and is associated with worse prognosis, both functional and anatomical [7,8].
Pars Plana Vitrectomy (PPV) was proposed as treatment for this entity, but initial results analyzed up to 2012 were discouraging [9,10]. Aiming to improve MH-MFS closure rates, Macular Buckling (MB) together with PPV was employed to counteract the tractional effect induced by the posterior staphyloma, showing excellent MH-MFS closure rates and halting progression towards a retinal detachment, but represents a highly complex procedure [11-14].
Recent studies show that the inverted Internal Limiting Membrane (ILM) flap technique, described in 2010 for MH [15], is highly efficient in closing MH in highly myopic patients without foveoschisis [15,16], and in patients with Macular Hole Retinal Detachment (MHRD) [15,17]. However, the cases of MH-MFS remain to be evaluated under this technique.
New findings in highly myopic eyes with retinoschisis, using enhanced vitreous imaging with Optical Coherence Tomography (OCT), immunocytochemistry and transmission electron microscopy, reveal a high prevalence of Vitreo Macular Interface (VMI) abnormalities, where the vitreous cortex tends to remain attached thus creating traction to the foveal area even in eyes with complete Posterior Vitreous Detachment (PVD) [14,18].
In this retrospective study, we re-evaluate PPV on the hypothesis that removing the VMI abnormalities and using the inverted ILM flap technique MH-MFS is a safe and efficient technique without the surgical complications associated with a MB.
Methods
This retrospective observational study was conducted, main inclusion criteria was the presence of MH-MFS documented by OCT. High myopia was considered as an axial length of >26.5 mm, full-thickness MH was defined as a foveal break of all cellular retinal layers (connecting VMI abnormalities were not considered to define the presence of a MH) and foveoschisis as an enlargement of the retinal thickness due to separation of the neurosensory retina in two or more layers.
Exclusion criteria included: lamellar MH-MFS (cases in which outer retinal layers were detected within the area of the hole), MHRD (defined by us as a neurosensorial retinal detachment of more than 3000 μm with a base to hole ratio >10:1), previous retinal surgery and severe optic nerve changes.
Surgical Procedure
All patients underwent standard 3-port 23-gauge PPV performed by the same surgeon (C.M.). ILM staining was performed with Brilliant Blue, 0.025% (Tissue blue; DORC International, Netherlands). Visualization of the macular area was achieved using macular contact lens (Macular Window; Advanced Visual Instruments, New York). Pre-ILM material (or VMI abnormalities) were removed using an ILM forceps in a circular manner. After this, if needed, repeated staining was conducted until a homogeneous staining was obtained, followed by the grasping and peeling of the ILM in a circular manner for approximately two-disc diameters around the MH with the use of ILM forceps. An inverted foveal-sparing trimmed flap of ILM attached to the periphery was placed over the macular hole. This step was performed under liquid perfluorocarbon (PFCL) to maintain stability of the flap. A drop of platelet concentrate was used as adhesive substance to maintain the flap in place. Fluid-air exchange was then performed and finally, a sulfur hexafluoride (SF6) tamponade was used. Patients were advised to maintain a prone position for 5 days.
Evaluation
All patients were examined before macular surgery and had a minimum of 6 months follow up after surgery. At each visit, patients underwent a complete ophthalmologic examination, including refraction, Best-Corrected Visual Acuity (BCVA) using Early Treatment Diabetic Retinal Study (ETDRS) chart, fundus photography with a mydriatic fundus camera (TRC-50X type IA; Topcon, Tokyo, Japan), measurement of axial length by optical biometry (IOL Master 500; Carl Zeiss Meditec AG, Jane, Germany), and spectral-domain OCT (SD-OCT) using the Cirrus HD-OCT 5000 system (software version 11.5.1) (Carl Zeiss Meditec, Dublin, CA).
The primary outcome was anatomic macular hole closure with resolution of the foveoschisis, defined as the resolution of the full thickness neurosensory defect over the fovea and at least a 50% decrease in retinal thickness in the central horizontal 6mm HD Line Raster. Secondary outcomes were changes in BCVA, preoperative Hole Form Factor (HFF) and time for foveoschisis resolution.
Statistical Analysis
Numerical variables were analyzed using paired t-student test. Qualitative variables are expressed as frequencies and percentages and were analyzed using chi-square test. Associations between the final BCVA and the clinical parameters were examined by univariate analysis. Statistical analysis was performed in R (version 4.0.3; R Core Team, Vienna, Austria) using a p-value <0.05 for statistical significance
Results
Results from 12 eyes of 12 consecutive patients (9 female, 3 male) were analyzed. Their mean age was 59.3 ± 11.4 years (range: 44-77 years). The mean Axial Length (AL) was 32.2 mm (range: 27.7-35.2 mm). 8 eyes were pseudo phakic, none were aphakic and cataract surgery was performed simultaneously with PPV in one patient. Postoperative follow upranged between 6 to 52 months with a median period of 17 months. ILM peeling was performed in all cases, while successful foveal-sparing ILM flap was obtained in 10 (83.3%) cases. No choroidal detachments or intraocular hemorrhages were reported intra or postoperative.
Closure of MH at the first month follow-up visit was achieved in 11 (90.9%) patients. All these cases remained closed during the follow up. One case failed to close by the first month and was re intervened. The ability to create or not a foveal sparring ILM-flap didn’t influence the MH-closure rate or the resolution of the foveoschisis.
Foveoschisis resolved completely in 9 (75%) patients, resolved partially in 2 (16,7%) patients, and persisted in 1 (8,3%) patient. In those patients in which foveoschisis resolved completely, resolution was seen by the first month in half of patients while the remaining 25% resolved within the first year. Partial resolution or non-resolution of foveoschisis was not associated with a worse visual outcome.
Main BCVA improved from 0.59 ± 0.26 logMAR (Snellen 20/77) preoperatively to 0.35 ± 0.25 log MAR (Snellen 20/44) with statistical significance (p=0.004). BCVA improved postoperatively in 9 patients (75%), two remained unchanged and worsened in one patient. None of the preoperative characteristics of the MH-MFS (Table 2) were found to correlate with the visual or anatomical success.
Variable
Data
Sex
Male
3 (25%)
Female
9 (75%)
Age, years
59,3 ± 11,4 (44 - 77)
Axial Length, mm
31,33 ± 2,74 (27,7 - 35,2)
BCVA (log MAR)
Preoperative
0.59 ± 0.26 (0.05 - 0.80)
Postoperative
0.35 ± 0.25 (0.10 - 0.80)
Delta
0.22
pa
0.0039
Follow up (months)
19.5 ± 13.15 (6 - 52)
Lens Status
Phakic
4 (33.3%)
Pseudophakia
8 (77.7%)
Surgical Technique
Concomitantlens surgery
1 (8.3%)
Successful ILM Flap
10 (83.33%)
a t test
Table 1: Demographic and ophthalmic characteristics of participants.
Variable
Median
MH base diameter (mm)
749 ± 725.9
MH mínimum diameter (mm)
277 ± 171.7
MH height (mm)
582 ± 152
Maximum height of retinoschisis
387 ± 140.4
Table 2: Preoperative Characteristics of MH-MFS.
A VMI-ILM residue was identified on the post-operative OCT scan of the patient in which the MH failed to close. This patient was re intervened on the first month follow-up visit, creating a new ILM Flap under PFCL and MB and achieved closure one month after re intervention. Another patient in which the MH closed within first month and BCVA improved significantly shows a persistent shallow subretinal fluid in absence of choroidal neovascularization.
Discussion
MH are a potential cause of vision loss in highly myopic patients and tend to appear in earlier ages than in emmetropic patients. According to Kobayashi et al, in eyes with an AL > 26 mm, MH appears at a mean age of 52 years. In patients with an AL between 23-25 mm, mean age is 64 years, while in AL < 23 mm, mean age of MH presentation is 70 years [4]. Patients may be initially asymptomatic and biomicroscopic diagnosis can be challenging due to vitreous opacities and Retinal Pigmentary Epithelium (RPE) disturbances [5]. Therefore, OCT is crucial for diagnosis, classification, and postoperative closure confirmation.
For many years, surgical resolution of myopic MH has been challenging. PPV was first proposed in 1982 as a solution for macular detachments with MH by Gonvers and Machemer [6], but results never exceeded 50% closure rates [1,8]. In 2006, a subtype of MH was described in high myopia, associated with macular retinoschisis that resulted in worse anatomic and functional results [1]. Three different series between 2006 and 2012 demonstrated anatomical closure of MH in high myopia between 25 and 50%, with functional improvement between 20-40% [1-3]. Sudaet al demonstrated that eyes with higher AL where more prone to closure failure, being most significant in eyes with AL > 30 mm and in eyes with retinoschisis-like thickening [3].
Trying to explain these results, Ikuno and Tano hypothesized that the retina is too stretched in these eyes and the rigidity of the inner layers may induce an enlargement of the macular hole when trying to readapt the innermost part of the retina to a larger arc of the posterior eye wall contour [1]. This led to a new course in the surgical approach towards MB surgery alone or combined with PPV for MH-MFS, as the buckle can counteract the pulling effect of the staphyloma and help relax the retina, increasing MH closure rate. By 2018, Burés-Jelstrup et al, showed a success rate of 100% in MH closure and BCVA gains in 80% of patients using combined PPV with MB, whereas Cacciamani et al showed similar results using MB without PPV [7-9].
However, long term follow-up of patients with MB also suggested that excessive compression under the choroidal vessels could induce RPE changes like atrophy and choroidal neovascularization [10,11]. Furthermore, MB surgery has failed to become a widely spread surgical option, since the technique is considered complex by most vitreoretinal surgeons and severe complications have been described, such as muscle injury and choroidal detachment secondary to vortex vein damage.
The lack of elasticity of the ILM due to its type IV collagen composition in a centrifugally growing eye and the abnormalities in the VMI in high myopia have refocused the pathogenesis of MTM [12,13]. Contrary to previous concepts, enhanced vitreous imaging optical coherence tomography (EVI-OCT) showed that a complete PVD, defined clinically as a complete Weiss Ring, does not represent a detachment of the cortical vitreous of the ILM in the macular area in high myopia. Residual cortical vitreous adhesion to the macular area can be detected in up to 70.9% of patients with high-myopic retinoschisis and a “complete” PVD [14]. These VMI abnormalities can sometimes be detected preoperatively by OCT as a hyper reflective substance at the border of the hole of MH-FS and postoperatively, in those eyes who failed to close the MH-MFS (Figure 2).
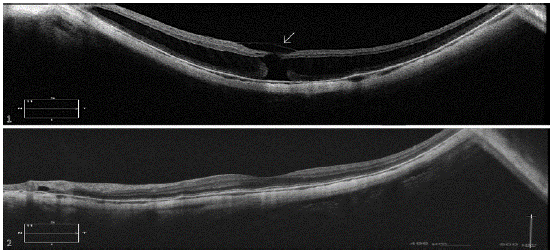
Figure 1: Preoperative OCT of MH-MFS cases. Cases 2, 6, 7 and 11 shows presence of a VMI abnormality in the apex of the hole. However, no neurosensorial retina is observed within the abnormality and there is no retinal tissue in the base of the hole. Case 12 shows a base of 2614 mm and a base to hole ratio of 9, hence it cannot be considered a MHRD.
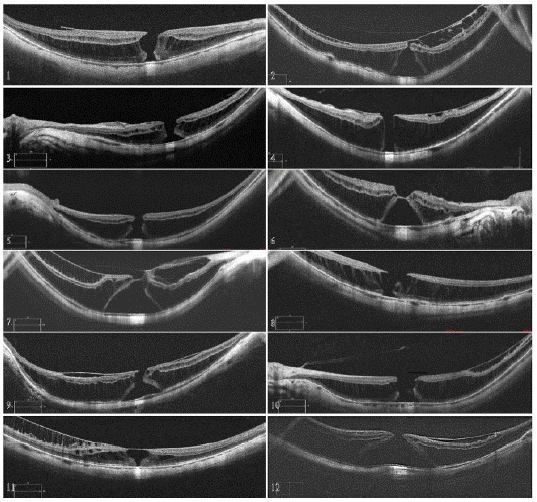
Figure 2: Case 1: postoperative OCT of the patient that failed to close in the first intervention. Persistent VMI (arrow) can be observed on top of the MH despite ILM peeling was described during surgery. Case 2: postoperative OCT of a successful case. This patient improved BCVA from 20/100 to 20/25.
The VMI in MFS as seen by immunohistochemistry and electron microscopy reveal masses of thick collagen strands in the vitreous that adhere firmly to a pathological thinner ILM than the one seen in epiretinal membranes [18]. This finding correlates clinically to the difficulty found to appropriately stain the ILM in MFS cases, but also represents a new insight when evaluating OCT, as there are MH-MFS in which the presence of VMI abnormalities act as a roof to the MH but there is no neurosensorial retina involved (Cases 3,7,8,12). These cases must be differentiated from lamellar MH-MFS as visual results tend to be better due to the presence of outer retinal layers in the foveal space [19].
Surgical management of MH-MFS cases require a series of considerations. First, good visualization of the macular area is crucial, as RPE changes, atrophic zones and a higher plane of work that expected due to foveoschisis may be encountered during surgery. In our opinion, using a contact macular lens provides a better contrast and stereopsis.
Furthermore, Brilliant Blue frequent restaining of the macular area is key to identify the ILM, as the first staining usually dyes in a patchy irregular pattern, corresponding to the VMI residues in the macular area. These VMI residues should be removed either using a ILM forceps or a flexible nitinol loop. After this step, restaining should show the typical regular dye pattern of the ILM. PFCL and platelet concentrate might come in handy for creating and fixing the ILM flap, especially in bigger holes. However, we believe this is a matter of surgical preference and doesn’t influence the success rate if the ILM has been correctly peeled. Supplementary video is provided to demonstrate the surgical steps.
MH-MFS must be differentiated surgically from just MFS and MHRD in the in MTM spectrum. MH-MFS requires a more challenging surgery than PPV for MFS as a more careful approach must be taken to find and relieve traction around the hole. In the case of MHRD, we suggest the definition criteria of a >3000 m neurosensorial detachment with a base to hole ratio >10:1, as this marks, in our experience, a more complex ILM peeling due to the mobility of the detached retina and the need to aspirate fluid through the hole to achieve success.
Conclusion
Our evidence suggests that PPV, with careful attention to removal of the VMI abnormalities and the creation of an inverted ILM flap, is sufficient to relieve the traction in MH-MFS and achieve visual and anatomical success. The presence of a VMI abnormality does not discard the presence of a MH-MFS. MH-MFS must be differentiated from MHRD as they comprise different levels of surgical complexity.
Ethics Approval and Consent to Participate
According to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee at Instituto de Microcirugía Ocular (IMO). Written consent was obtained from all patients.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Authors Contributions
GRV: Formal-analysis, Writing- Original draft preparation. FOC: Data curation, Writing- Reviewing and Editing. ABJ: Writing- Reviewing and Editing. CM: Conceptualization, Visualization, Supervision.
References
- Ikuno Y, Tano Y. Vitrectomy for Macular Holes Associated With Myopic Foveoschisis. American Journal of Ophthalmology. 2006; 141: 774-6.
- Jo Y, Ikuno Y, Nishida K. Retinoschisis: a predictive factor in vitrectomy for macular holes without retinal detachment in highly myopic eyes. Br J Ophthalmol. 2012; 96: 197-200.
- Suda K, Hangai M, Yoshimura N. Axial Length and Outcomes of Macular Hole Surgery Assessed by Spectral-Domain Optical Coherence Tomography. American Journal of Ophthalmology. 2011; 151: 118-27.e1.
- Kobayashi H, Kobayashi K, Okinami S. Macular hole and myopic refraction. British Journal of Ophthalmology. 2002; 86: 1269-73.
- Coppé AM, Ripandelli G, Parisi V, Varano M, Stirpe M. Prevalence of asymptomatic macular holes in highly myopic eyes. Ophthalmology. 2005; 112: 2103-9.
- Gonvers M, Machemer R. A new approach to treating retinal detachment with macular hole. Am J Ophthalmol. 1982; 94: 468-72.
- Alkabes M, Padilla L, Salinas C, Nucci P, Vitale L, Pichi F, et al. Assessment of OCT measurements as prognostic factors in myopic macular hole surgery without foveoschisis. Graefe's Archive for Clinical and Experimental Ophthalmology. 2013; 251: 2521-7.
- Alkabes M, Mateo C. Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefe's Archive for Clinical and Experimental Ophthalmology. 2018; 256: 863-77.
- Cacciamani A, Lazzeri S, Rossi T, Scarinci F, Parravano M, Ripandelli G, et al. ADJUSTABLE MACULAR BUCKLING FOR FULL-THICKNESS MACULAR HOLE WITH FOVEOSCHISIS IN HIGHLY MYOPIC EYES: Long-Term Anatomical and Functional Results. Retina. 2016; 36: 709-16.
- Burés-Jelstrup A, Alkabes M, Gómez-Resa M, Rios J, Corcóstegui B, Mateo C. Visual and anatomical outcome after macular buckling for macular hole with associated foveoschisis in highly myopic eyes. British Journal of Ophthalmology. 2014; 98: 104-9.
- Mateo C, Burés-Jelstrup A. MACULAR BUCKLING WITH ANDO PLOMBE MAY INCREASE CHOROIDAL THICKNESS AND MIMIC SEROUS RETINAL DETACHMENT SEEN IN THE TILTED DISK SYNDROME. Retin Cases Brief Rep. 2016; 10: 327-30.
- Vielmuth F, Schumann RG, Spindler V, Wolf A, Scheler R, Mayer WJ, et al. Biomechanical Properties of the Internal Limiting Membrane after Intravitreal Ocriplasmin Treatment. Ophthalmologica. 2016; 235: 233-40.
- Shimada N, Tanaka Y, Tokoro T, Ohno-Matsui K. Natural course of myopic traction maculopathy and factors associated with progression or resolution. Am J Ophthalmol. 2013; 156: 948-57.e1.
- Song M, Shen M, Zhou Y, Zheng K, Zhai Y, Xiao M, et al. Observation of Vitreous Features using Enhanced Vitreous Imaging Optical Coherence Tomography in Highly Myopic Retinoschisis. Retina. 2019; 39: 1732-41.
- Chatziralli I, Machairoudia G, Kazantzis D, Theodossiadis G, Theodossiadis P. Inverted internal limiting membrane flap technique for myopic macular hole: A meta-analysis. Surv Ophthalmol. 2021; 66: 771-80.
- Bové Álvarez M, Sabaté S, Gómez-Resa M, García-Arumí J. Anatomical and Visual Outcomes of Inverted Internal Limiting Membrane Flap Technique Versus Internal Limiting Membrane Peeling in Myopic Macular Hole without Retinal Detachment: A Preliminary Retrospective Study. Retina. 2020; 40: 233-40.
- Xu Q, Luan J. Vitrectomy with inverted internal limiting membrane flap versus internal limiting membrane peeling for macular hole retinal detachment in high myopia: a systematic review of literature and meta-analysis. Eye. 2019; 33: 1626-34.
- Vogt D, Stefanov S, Guenther SR, Hagenau F, Wolf A, Priglinger SG, et al. Comparison of Vitreomacular Interface Changes in Myopic Foveoschisis and Idiopathic Epiretinal Membrane Foveoschisis. Am J Ophthalmol. 2020; 217: 152-61.
- Zhang Z, Wei Y, Jiang X, Zhang S. PARS PLANA VITRECTOMY AND WIDE INTERNAL LIMITING MEMBRANE PEELING WITH PERFLUOROPROPANE TAMPONADE FOR HIGHLY MYOPIC FOVEOSCHISIS-ASSOCIATED MACULAR HOLE. Retina. 2017; 37: 274-82.