
Research Article
J Ophthalmol & Vis Sci. 2023; 8(2): 1078.
Randomized Study of Intravitreal Injection of Bevacizumab in the Treatment of Persistent Uveitic Macular Edema
Bouslamti A*, Bardi C, Boujaada A, Rostoum L, Hasnaoui I, Serghini L, Elhassan A and A Berraho
Ophthalmology B, Ibn-Sina University Hospital, Rabat, Morocco
*Corresponding author: Bouslamti AOphthalmology B, Ibn-Sina University Hospital, Rabat, Morocco
Received: January 19, 2023; Accepted: March 13, 2023; Published: March 20, 2023
Abstract
Objective: To assess the efficacy and safety of Bevacizumab IVT in the treatment of persistent uveitic macular edema
Methods: This is a prospective randomized interventional study of 20 eyes of 20 macular edema patients with different types of uveitis in remission but persistent to conventional therapy who were treated with Bevacizumab IVT, with short- and medium-term results collected and analyzed.
Results: The improvement in VA was significant as early as week 12, and at 12 months reached 80%. The mean logMAR VA was initially 0.78. After 1 year, it decreased to 0.57. 16 patients (80%) had at least 2 lines of VA gain at 1-year follow-up. Reinjection was required in the majority of cases. 8 patients (40%) had a 2nd IVT of Bevacizumab and 5 patients (25%) had 3 IVT of Bevacizumab.
After one or more IVT of Bevacizumab, the improvement of at least 2 lines of VA at 12, 24 weeks and 1 year were 45%, 60% and 80% respectively. 65% of our patients had a central macular thickness <300 μm at 1 year follow-up. The mean central macular thickness was initially 546.55 μm, after 1 year of follow-up, the central macular thickness was 369.95 μm. After 3 months of follow-up, there was a reduction of at least 60 μm, and after 1 year of follow-up, the reduction in central macular thickness was at least 100 μm and this affected 65% of patients. No serious ocular or systemic side effects were observed.
Conclusion: These results demonstrate that intravitreal injection of Bevacizumab in the treatment of persistent uveitic macular edema well tolerated, with short- and medium-term improvement in visual acuity and central macular thickness in a significant number of cases.
Keyswords: Persistent macular edema; Uveitis; IVT of Bevacizumab; Visual acuity; Central macular thickness
Introduction
Uveitis is a group of all inflammatory ocular diseases, a major cause of ocular morbidity and the fifth leading cause of legal blindness worldwide [1]. Macular edema can complicate anterior, intermediate, posterior uveitis and panuveitis of very different causes, including infectious or autoimmune inflammatory [2,3]. It is the major cause of significant and permanent visionloss in uveitis [4]. Medical management of uveitic macular edema includes topical and systemic Nonsteroidal Anti-Inflammatory drugs (NSAI), local and systemic corticosteroids, systemic carbonic an hydrase inhibitors, and systemic immunosuppressants [5-7]. Uveitic macular edema can be resistant or unresponsive to these therapeutic modalities and persist despite successful control of ocular inflammation, it is said to be "refractory," and therefore warrants the investigation and use of different treatment options for this entity [8]. Part of the treatment is represented by intravitreal injections of Anti-VEGF, whose results will be evaluated in the short and medium term in the treatment of refractory uveitic macular edema, both in terms of efficacy and tolerance and side effects, as well as a review of the literature.
The purpose of this study is to provide preliminary efficacy data to determine whether intravitreal injection with Bevacizumab can be considered for the treatment of macular edema secondary to uveitis and resistant to conventional therapy.
Material and Methods
The study we conducted is a prospective randomized, monocentric interventional study in patients followed and treated for uveitic macular edema refractory to treatment involving 20 eyes of 20 patients, within the department of Ophthalmology B of the Hospital of Specialties in Rabat extending from January 2021 to February 2022, with the aim of obtaining data over one year allowing a 12-month hindsight.
General criteria were included in our study: male or female, not pregnant, at least 18 years of age at the time of consent; the patient is able to understand and sign the informed consent form; the patient is able to attend the scheduled consultations, treatment schedule and paraclinical examinations; the patient has no contraindication to intravitreal injection of Bevacizumab.
As well as ophthalmologic criteria, represented by: macular edema; uveitic macular edema; macular edema recalcitrant to any topical general/periocular anti-inflammatory treatment; subconjunctival, subtenonial, general and/or intravitreal or having received systemic treatment with carbonic anydrase inhibitor or immunosuppressant.
Exclusion criteria were: contraindications to Bevacizumab; pregnant or lactating women; women of childbearing potential who are unwilling or unable to use contraception; lost to follow-up or uncooperative patients who refuse to comply with the study protocol; associated ocular disease that limits visual potential.
Results
Epidemiologic Analysis
We collected 20 eyes of 20 patients with refractory uveitic macular edema, which were analyzed prospectively: All patients had been previously diagnosed with uveitis of varying cause. Uveitis was in remission at the time of injection in all patients. In all patients, macular edema was confirmed by biomicroscopy and macular OCT. All 20 eyes received at least one intravitreal injection of Bevacizumab, with retreatment dependent on the response to the initial IVT. No patient received additional treatment during the 1-year follow-up period.
There were 12 men (60%) and 8 women (40%) in the study.
The overall mean age of our patients at the start of IVT Bevacizumab treatment was 34.45 years.
The most common pathologies found in our study were Behçet’s disease (20%), idiopathic intermediate uveitis (15%), juvenile idiopathic arthritis (15%), followed by sarcoidosis (10%), VKH disease (10%) and MS (10%) and lastly Birdshot (5%), Irvine Gass (5%), Tuberculosis (5%) and post traumatic uveitis (5%).
Macular edema was found in 45% of cases in posterior uveitis, 30% of cases in panuveitis and only in 10% of cases in the remaining types of uveitis (anterior uveitis, intermediate uveitis, parsplanitis).
Concerning the type of macular edema
- Cystoid macular edema is the most frequently encountered anatomical form of interest in 70% of our cases.
- 3 patients had a diffuse macular thickening without cystoid character (cystic pockets) and without sub-retinal fluid.
- 3 other patients, i.e. 15% of the cases, presented a DSR with an increase of the CMT (central macular thickness)
Concerning the age of macular edema and treatment received
A bolus of methylprednisolone relayed by oral prednisolone was administered in 80% of our patients, who received it at least 3 months before Bevacizumab IVT, followed or not by immunosuppressant. Only 3 patients received locoregional injections of CTC, there is only one case that was satisfied with a topical injection with oral administration of IAC (Irvine Gass).
Effectiveness Analysis - Visual Acuity
The analysis showed that the probability of VA improvement increased significantly from 12 weeks, reaching 70% at 24 weeks.
The mean initial logMAR VA was 0.78. At 1 year, the mean logMAR visual acuity was 0.57.
16 patients (80%) presented a VA gain of at least one line at the time of their last consultation (12 months). 4 of our patients, or 20% of cases, showed no improvement in their VA during the entire year of follow-up.
As shown in Table 3, the improvement in VA of at least 2 lines at 12, 24 weeks and 1 year after one or more injections was 45%, 60% and 80%, respectively.
Patient
Sex
Age
Diagnostic
ANATOMIC SITE
1
M
38
Behcet
panuveitis
2
F
42
SEP
posterior uveitis
3
M
52
idiopathic
Parsplanitis
4
M
18
JIA
anterior uveitis
5
M
28
Behcet
panuveitis
6
F
45
sarcoidosis
panuveitis
7
M
42
Tuberculosis
posterior uveitis
8
F
32
VKH
posterior uveitis
9
F
38
SEP
posterior uveitis
10
M
19
JIA
anterior uveitis
11
M
54
IrvineGass
posterior uveitis
12
M
38
idiopathic
Parsplanitis
13
M
36
Behcet
panuveitis
14
F
46
VKH
posterior uveitis
15
F
34
idiopathic
panuveitis
16
M
20
JIA
intermediate uveitis
17
F
42
sarcoidosis
posterior uveitis
18
M
25
Behcet
panuveitis
19
F
22
Birdshot
posterior uveitis
20
M
18
post traumatic uveitis
posterior uveitis
Table 1: Patient data, uveitis entities and anatomical site.
Patient
Sex
Age
Etiology
Seniority of the MO
Treatment received <3monthsIVT
1
M
38
Behcet
6 months
Bolus o fCTC+Azathioprine
2
F
42
SEP
4 months
Bolus of CTC 3 days
3
M
52
Idiopathic
7 months
Sub-conjunctival injection CTC
4
M
18
JIA
6 months
Bolus of CTC+relayperos
5
M
28
Behcet
9 months
Bolus of CTC+relayperos
6
F
45
Sarcoïdosis
9 months
Bolus of CTC+relayperos
7
M
42
Rétinite
virale12 months
Bolus of CTC undercover
Antiviral8
F
32
VKH
18 months
Bolus of CTC 5 days+relay peros
+cyclophosphamide9
F
38
SEP
5 months
Bolus of CTC 3 days
10
M
19
JIA
6 months
Bolus of CTC+relayperos
11
M
54
IrvineGass
14 months
Topical NSAI+Acétazolamide
peros12
M
38
Idiopathic
7 months
Sub-tenon injection CTC
13
M
36
Behcet
9 months
Bolus of CTC+relayperos
14
F
46
VKH
20 months
Bolus of CTC+relayperos
15
F
34
Idiopathic
7 months
Bolus of CTC+relayperos
16
M
20
JIA
9 months
IVTof triamcinolone
17
F
42
Sarcoïdosis
12 months
Bolus of CTC+relayperos
18
M
25
Behcet
9 months
Bolus of CTC+relayperos+
Azathioprine19
F
22
Birdshot
10 months
Bolus of CTC+relayperos
20
M
18
post traumatic uveitis
9 months
Bolus of CTC+relayperos
Table 2: Age of macular edema and treatments received at least 3 months before Bevacizumab IVT.
Patient
VA pre IVT
VA at 1 Month of IVT
VA at 3 Month
VA at 6 Month
VA at 12 Month
1
0,6
0,52nd IVT
0,4
0,43rd IVT
0,1
2
0,2
0,2
0,2
0,2
0,2
3
0,7
0,62nd IVT
0,53rd IVT
0,4
0,4
4
0,4
0,4
0,42nd IVT
0,2
0,1
5
0,7
0,62nd IVT
0,3
0,2
0,2
6
0,5
0,6
0,6
0,6
0,5
7
0,5
0,5
0,5
0,32nd IVT
0,3
8
0,8
0,8
0,5
0,2
0,2
9
0,1
0,1
0,1
0,1
0,1
10
0,5
0,42nd IVT
0,4
0,33rd IVT
0,3
11
1,7
1
0,72nd IVT
0,53rd IVT
0,2
12
1,7
0,7
0,62nd IVT
0,6
0,6
13
0,4
0,4
0,2
0,22nd IVT
0,2
14
1,7
1,7
1,7
1,7
1,7
15
1,7
12nd IVT
0,93rd IVT
0,8
0,8
16
0,3
0,3
0,22nd IVT
0,1
0,1
17
1,7
0,92nd IVT
0,9
0,9
0,9
18
0,3
0,3
0,3
0,22ème IVT
0,1
19
0,4
0,4
0,4
0,4
0,2
20
0,8
0,8
0,7
0,52nd IVT
0,1
Table 3: LogMar AV of patients treated and retreated with IVT Bevacizumab during 1-year follow-up.
Patient
EMCpréIVT
EMCà3 mois
EMCà6 mois
EMCà 1an
1
646
328
315
179
2
334
230
236
229
3
473
460
390
355
4
330
300
288
251
5
469
380
230
214
6
480
480
480
480
7
664
550
500
350
8
514
520
316
258
9
232
232
232
232
10
550
489
347
296
11
580
470
360
232
12
728
704
693
676
13
364
360
340
203
14
1122
1123
1123
1122
15
795
675
593
521
16
340
235
235
235
17
937
937
935
935
18
343
312
279
216
19
461
403
403
211
20
669
498
300
204
Table 4: Evolution of the CME during the follow-up period.
The best visual acuity was obtained after 1 year of follow-up.
After an initial IVT with Bevacizumab, patients with a positive response, defined as an increase of at least one VA line, were retreated after a minimum of 4 weeks.
8 patients received a second IVT of Bevacizumab or 40% of cases and only 5 patients who received 3 IVT of Bevacizumab or 25% of cases.
Patients who did not respond positively to IVT did not receive a second injection.
Patient 6 showed a worsening of the VA in the injected eye 1 month after IVT, due to the appearance of an epiretinal membrane with obvious traction, which required vitrectomy with peeling of the membrane (Figure 3).
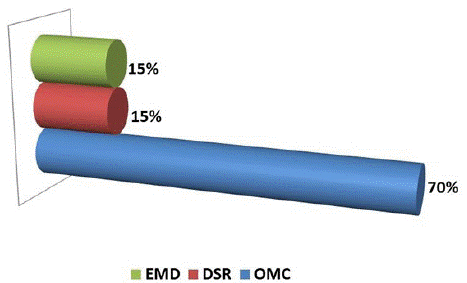
Figure 1: Distribution of MO according to their anatomical characteristics.
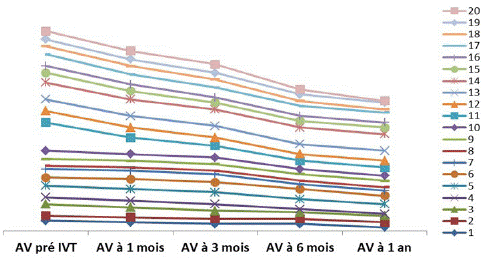
Figure 2: VA gain in LogMar over one year of follow-up.
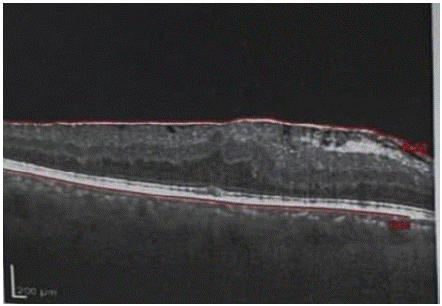
Figure 3: Patient 6 who developed MEM during follow-up.
Central macular thickness (CMT) in μm
A statistically significant change in CMT was noted during follow-up:
At 1 year of follow-up, 65% of patients had a CMT of less than 300 μm and 55% had a CMT of less than 250μm.
2 patients had increased and stable macular thickness after 1 year of follow-up (Patients 14 and 17 who had a CMT of 1122 μm and 935 μm respectively) (Figures 41,44)
The average CMT was initially 546.55 μm. After 1 year of follow-up, the CMT was 369.95 μm
The improvement in CMT was statistically significant at 3 months and 1 year of follow-up.
After 3 months of follow-up, there was a reduction of at least 60 μm, and after 1 year of follow-up, the reduction in CMT was at least 100μm and this affected 65% of patients.
Our results show a reduction in CMT from 60 μm to 100 μm or more with a significant improvement in VA of 0.4 logMAR or 3 lines on the Monoyer scale after 1 year of follow-up.
VA was not completely correlated with CMT. We take the example of patients 2, 19, and 20 who had a normal CMT, while their VA (0.2; 0.2 and 0.1 logMAR, respectively) remained below 0 logMar or 10/10 in Monoyer.
Favorable responses to Bevacizumab in the following patients: 1,2, 4, 5, 11, 13, 16, 18, 19, 20.
No significant ocular or systemic side effects were observed with Bevacizumab IVT in our study. No patient developed ocular hypertension or endophthalmitis after injection.
There was no evidence of recurrence of uveitis after Bevacizumab IVT. In addition, no thromboembolic events were recorded in our patients during the follow-up period.
VA and CME results were favorable in younger patients (Patient 1, for example), and/or in patients with cystoid macular edema whose thickness did not exceed 400 μm and whose the integrity of the photoreceptor line was respected.
Discussion
The response of uveitic macular edema (VA + CMT) to treatment with Bevacizumab injection differs according to the authors. The different responses to anti-VEGF therapy can be explained by the different variations in the etiopathogenesis of uveitic macular edema [9].
Cordero Coma and al. treated 13 eyes of 13 patients with ME secondary to uveitis, resulting in significant loss of visual acuity, persistent to medical treatments. The use of intravitreal injection of Bevacizumab showed promising results, with improved VA and CMT in 62% of patients after a single injection. The chance of VA improvement increased gradually by week 6, reaching 81% by week 14 [9].
According to the same authors, the injection of Bevacizumab in uveitic ME is more advantageous than injection of corticosteroids, as well as less likely to cause glaucoma or cataract. Bevacizumab is prepared from a preservative-free solution and also contains no known retinal damaging ingredients. None of the patients in this study had myodesopsias with Bevacizumab [9]. Nevertheless, it has several drawbacks, including a shorter half-life and reduced anti-inflammatory effect in the vitreous. Although no patients required repeat injections in this study, the small number of patients included and the short follow-up limit the ability to judge the need for reinjection [9].
Fine and al showed in a study the relationship between uveitic ME and the concentration of angiogenic factors in the aqueous humor (HA) and blood of patients. In this cross-sectional study, VEGF levels were measured by enzyme-linked immunosorbent assays in the HA of patients with uveitis. Therefore, these authors found that VEGF is a potential target in the management of uveitic ME [10].
K Weiss and al studied 11 eyes in 9 patients with uveitic ME treated with 1.25 mg Bevacizumab injection into the vitreous. The 1.25 mg dose was used because the vitreous VEGF concentrations observed by K. Weiss in patients with uveitis were similar to those in patients with exudative AMD. 1.25 mg is the most commonly used dose [11].
He concluded that the beneficial effects of intravitreal Bevacizumab were transient. While 9 eyes showed at least 2 lines of visual enhancement during the first 2 weeks, only 3 eyes maintained this visual enhancement during the follow-up period. Favorable responses to retreatment indicate that the effect of Bevacizumab is insufficient. According to the same author, the main limitation of this study is that it included a small number of patients and a larger study is needed [11].
29 eyes of 27 patients with uveitic macular edema complicating uveitis of various causes were studied and analyzed according to RENE A and al, with a follow-up of one year. 13 patients had a single intravitreal injection of Bevacizumab, 6 patients required a 2nd injection, with a mean logMAR visual acuity of 0.42. The average CMT decreased from 383.66 μm to 294.32 μm over the course of one year. The success of Bevacizumab injection in the treatment of uveitic ME is related to the fact that uveitis was in a lull in all patients before ME treatment [12].
LOTT and al. also retrospectively analyzed the effect of intravitreal injection of Bevacizumab on 13 eyes of 11 patients, with an average follow-up of 13 months. Each patient received between one and four IVTs (average of 2). After 6 months, 40% of patients improved in VA, 20% had no change, and 40% had worsening VA [13].
MACKENSEN and al. conducted a study involving 11 eyes of 10 patients, with 72% of patients having a favorable response 1 month after Bevacizumab injection and a significant reduction in CMT, while 4 patients had no improvement in VA [14].
Mirshahi and al. treated 12 patients with uveitic ME resistant to one or two IVTs of Bevacizumab but only in Behçet’s disease, with a mean follow-up of 4 months. VA improved in 58% of patients, remained stable in the remaining cases, while there was no significant change in CMT before and after treatment [15].
FARIBA GHASSEMI studied 19 patients and followed them for 6 months. 15 of them had ME due to Behçet’s disease and received an average of 3 IVT of Bevacizumab without statistically significant improvement in visual acuity and central macular thickness [16].
In our study, we collected 20 eyes of 20 patients with persistent uveitic ME. These patients constitute a cohort at a tertiary referral center, usually representing the most severe form of uveitis.
Our current study evaluates the short- and medium-term efficacy of Bevacizumab IVT in 20 patients with refractory uveitic ME, with or without the need for repeated injections.
All patients received at least one IVT of Bevacizumab, 8 eyes received a 2nd injection, and only 5 who needed 3 IVT to restore macular structure and visual function. The criterion for retreatment is a CMT maintained > at 250 μm.
We showed significant improvement in VA and CMT during a 1-year follow-up time. 80% of our patients showed at least 2 lines of VA gain, with the achievement of better VA gain and a mean LogMar VA of 0.57 at 1 year follow-up. The decrease in ME can explain the visual improvement in most cases. Indeed, 65% of the patients had a CMT <300 μm with a mean CMT that decreased from 546.55 μm to 369.95 μm after 1 year of follow-up.
VA is not always correlated with CMT, some authors report a moderate to strong correlation, and others like MACKENSEN show a very weak correlation [14]. In our study, the VA did not correlate with the CMT in 3 patients; the latter had a normal CMT with a VA that remained deteriorated.
4 of the patients (20%), showed no improvement in VA or CMT throughout the follow-up period.
Our results are consistent with those of the following studies: Cordero Coma [9] and RENE A [12].
These initial data suggest that Bevacizumab injection may be an attractive complementary option for the treatment of refractory uveitic ME.
Our results find that the off-label use of Bevacizumab in the treatment of uveitic ME is well justified and tolerated. Early initiation of treatment, before the onset of retinal fibrosis, is recommended to prevent irreversible photoreceptor damage.
The strengths of our study are the number of patients included and the follow-up period (1 year). However, longer studies with a much longer follow-up period are warranted. Further randomized controlled trials on this topic with comparative statistical measurements of functional and anatomical data on macular OCT are recommended. We emphasize the critical importance of remission of uveitis for all patients who have received such treatment for refractory uveitic ME.
Conclusion
ME is the 1st cause of blindness in patients with uveitis. It remains a redoutable complication and very difficult to manage despite the many therapeutic strategies available.
Uveitic ME may persist despite remission of uveitis. The numerous adverse effects associated with the chronic use of ocular corticosteroids (local and/or systemic) for the treatment of uveitic macular edema have led to the search for new therapeutic options.
The mechanism by which anti-VEGF monoclonal antibodies might be effective in the treatment of uveitic macular edema is a matter of speculation. Intravitreal Bevacizumab appears to be a useful and advantageous alternative in the treatment of persistent uveitic macular edema. This therapeutic alternative has the advantage of fewer side effects but has the disadvantage of a short duration of action, hence the need for repeated injections. In addition to the risks associated with any IVT, such as endophthalmitis and systemic passage of Bevacizumab.
Assessment and measurement of intravitreal VEGF levels in patients with uveitic macular edema provides a basis for determining intravitreal VEGF-A concentrations and justifies the treatment and injection doses of Bevacizumab.
References
- N Pogorzalek, I de Monchy, G Gendron, M Labetoulle. Uvéite et hypertonie: à propos de 103 patients Hypertony and uveitis: 103 cases of uveitis JFO. 2011; 34: 157-163.
- Tran VT, Auer C, Guex-Crosier Y, Pittet N, Herbort CP. Epidemiology of uveitis in Switzerland. Ocul Immunol Inflamm. 1994; 2: 169-176.
- Paivonsalo-Hietanen T, Tuominen J, Vaahtoranta-Lehtonen H, Saari KM. Incidence and prevalence of different uveitis entities in Finland. Acta Ophthalmol Scand. 1997; 75: 76-81.
- Rothova A. Medical treatment of cystoid macular edema. Ocul Immunol Inflamm. 2002; 10: 239-46.
- Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996; 80: 332-336.
- Schilling H, Heiligenhaus A, Laube T, Bornfeld N, Jurklies B. Long-term effect of acetazolamide treatment of patients with uveitic chronic cystoid macular edema is limited by persisting inflammation. Retina. 2005; 25: 182R188.
- El-Shabrawi Y, Mangge H, Hermann J. Anti-tumour necrosis factor alpha treatment in chronic recurrent inflammation of the anterior segment of the eye in patients resistant to standard immunomodulatory treatment. Ann Rheum Dis. 2003; 62: 1243-1244.
- Murphy CC, Greiner K, Plskova J, Duncan L, Frost A, et al. Neutralizing tumor necrosis factor activity leads to remission in patients with refractory noninfectious posterior uveitis. Arch Ophthalmol. 2004; 122: 845-851.
- Miguel Cordero Coma, Lucia Sobrin, Sumru Onal, William Christen, C Stephen Foster. Intravitreal Bevacizumab for Treatment of Uveitic Macular Edema Ophtalmology. 2008.
- Fine HF, Baffi J, Reed GF, Csaky KG, Nussenblatt RB. Aqueous humor and plasma vascular endothelial growth factor in uveitis-associated cystoid macular edema. Am J Ophthalmol. 2001; 132: 794-6.
- K Weiss, I Steinbrugger, M Weger, N Ardjomand, R Maier, et al. Intravitreal VEGF levels in uveitis patients and treatment of uveiticmacular oedema with intravitreal bevacizumab. Eye (Lond). 2009; 23: 1812-1818.
- Castañeda RAC, Giuliari, Gallagher MJ, Yilmaz T, Macdonell RE, et al. Intravitreal Bevacizumab in refractory uveitic macular edema: one-year follow-up. European Journal of Ophthalmology. 2009; 19: 622-629.
- Lott MN, Schiffman JC, Davis JL. Bevacizumab in inflammatory eye disease. Am J Ophthalmol. 2009; 148: 711-717.
- Mackensen F, Heinz C, Becker MD, Heiligenhaus A. Intravitreal bevacizumab (Avastin) as a treatment for refractory macular edema in patients with uveitis: a pilot study. Retina. 2008; 28: 41-5
- Mirshahi A, Namavari A, Djalilian A, Moharamzad Y, Chams H. Intravitreal bevacizumab (Avastin) for the treatment of cystoid macular edema in Behcet’s disease. Ocul Immunol Inflamm. 2009; 17: 59 64.
- Ghassemi F, Mirak SA, Chams H. Eye Research Center, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, Iran 2The Retina and vitreous surgery service, Farabi Eye Hospital, Tehran University of Medical Sciences, Tehran, Iran Characteristics of Macular Edema in Behcet Disease after Intravitreal Bevacizumab. 2017; IP: 73.92.237.176.