
Research Article
J Ophthalmol & Vis Sci. 2023; 8(2): 1080.
Novel Treatment Options for Corneal Edema
Schrage T1, Panfil C1,2, Schrage N1 and Fuest M1,2
¹Aachen Centre for Technology Transfer in Ophthalmology, Germany
²Department of Ophthalmology, RWTH Aachen University, Germany
*Corresponding author: Schrage TThomas Schrage, Aachen Centre for Technology Transfer in Ophthalmology, Karlsburgweg 9, D 52070 Aachen, Germany
Received: January 17, 2023; Accepted: March 13, 2023; Published: March 20, 2023
Abstract
Introduction: The current treatment of corneal edema is limited to hyperosmotic saline mixtures. Usually the therapy consists of treatment with hypertonic 5% NaCl eye drops or 6% eye ointment. However, these only remain on the cornea for a short time because they are quickly removed by the tear film and blinking. This problem is partly addressed by adding hyaluronic acid to some pharmaceuticals. In this work, Sovermol as a carrier oil supplied with osmotically active substances, honey, icing sugar, Substance C188 and Prevor® substance X were investigated in an experimental setting as therapeutics for corneal edema.
Material and Methods: In the Ex vivo Eye irritation Test (EVEIT), corneas can be examined under defined and stable biochemical conditions without blinking. Corneal edema can be simulated and examined using ultrasound pachymetry and Optical Coherence Tomography (OCT). Thus, we investigated the deswelling effect of the substances described above on 23 EVEIT corneas.
Results: The mixtures, examined in this study, showed a significant reduction of corneal edema especially mixtures of Sovermol with Substance C188 were very promising as a new approach to treatment of corneal edema. As reference substance for corneal edema treatment ODM5® was used. Other mixtures, especially Honey and Prevor® substance X in DOL showed a sufficient reduction of corneal edema, while also damaging the corneal epithelium. While the mixture of DOL and powder sugar showed an increased corneal thickness and damaged Epithelium.
Conclusion: The therapy of corneal edema with osmotically active substances as investigated here is a promising treatment for corneal edema. However, further pharmacological development is needed for application in patients.
Keywords: Cornea; Corneal edema; Fuchs’ endothelial dystrophy; Endothelial dysfunction
Key messages:
What is Known?:
- The cornea has different de-swelling mechanisms.
New Information:
- Medicinal de-swelling with suspensions of solids in oil is possible and sensible.
- The substance C188 is most effective in removing water from the cornea.
Introduction
In everyday clinical practice, acute and chronical corneal edema are a common problem. These can cause visual impairment and pain [1]. Acute corneal edema after phacoemulsification can lead to patient dissatisfaction. This is caused by the expectation of a quick Improvement in vision that is disappointed by the quickly healing corneal edema [2]. Part of the early therapy is to specifically remove water from the cornea to allow it to clear. Of course, the disease causing the corneal edema must be treated as casually as possible [3]. Especially in the treatment of chronic corneal edema, such as in Fuchs’ endothelial dystrophy or after endothelial trauma, we need therapeutic agents that reduce the corneal edema. Corneal edema is mostly caused by an imbalance between water inflow into the corneal stroma, for example due to defective endothelial pumping function and water loss by perspiration insensibilis. The therapeutic goal is to establish an equilibrium where the cornea has a normal water level and becomes transparent again [4].
In this work, known [5] and new substances and combinations to increase the drainage of water from the cornea will be investigated as a possible new therapy for corneal edema.
Hyperosmolar Therapeutics for the Treatment of Corneal Edema
The consideration of treating corneal swelling with hyperosmolar therapeutics is not new. It was used decades ago [6,7]. Therapy with 5% saline solution in form of drop or ointment is currently the major therapeutical option [8]. There are few systematic studies in this clinical context. In addition to the recent work by [9] the deswelling of the corneal stroma has been investigated primarily in the context of corneal culture. Specifically, dextran, HES [10] and chondroitin sulphate [11] have been investigated as deswelling therapeutics for corneal grafting. Dextranes, for example, are not suitable for the therapy of corneal edema when used on living humans due to their allergenic potential [12].
An essential characteristic of hyperosmolar saline solutions (Ocusaline®, Omnisorb®) approved and used clinically as medical devices is their aqueous base. However, the application of an aqueous solution places a heavy osmolar load on the epithelial barrier and the back-diffusion of tears and isoosmol are fluids becomes a problem after a typically short exposure time. This is comparable to the damage mechanism in Dry Eye Syndrome (DES) [13]. The ODM5® (TRB Chemedica AG, Feldkirchen Germany) eye drop solution, which has also been used for some years, takes this problem into account with a longer residence time on the eye due to the addition of hyaluronic acid.
To minimize the problem of the aqueous phase, we investigated a mixture of osmolar active substance in a suspension with a non-aqueous carrier substance. This pharmaceutical form is based on comparable models such as Glycocortisone® eye ointment (hydrocortisone, glucose, glycocortisone H eye ointment, Novartis, Basel, Switzerland), which was withdrawn from the market in 2002 due to production problems.
This solution contained a high osmolar medium, highly refined sugar and cortisone, in a Vaseline ointment. In this work we have experimentally evaluated similar suspensions, in a clear and liquid form, for their deswelling effect.
Material and Experiment
Ex Vivo Eye Irritation Test (EVEIT)
For this study we used 23 corneas in a non-animal-use model in which rabbit eyes (New Zealand white, slaughterhouse Lapinchen, Euskirchen) are enucleated 8 hours postmortem. These are nourished with an artificial aqueous humour, an iso-osmolar nutrient medium containing Earle´s salts and HEPES buffer [Eagle Minimal Essential Medium (MEM), HEPES buffer 5.8 g/L: both Biochrom GmbH, Berlin, Germany]. The initial thickness is determined using a Thorlabs OCT (Base unit Ganymed with Software Dev. Kit V3.0C software; Thorlabs, Dachau, Germany) or ACCUTOME® pachypen handheld pachymeter (A24-51003222 Phoenixville Pike, Building #50, Malvern, PA 19355 USA). We performed quality and vitality measurements as shown below. Quality criterion for using a cornea in this work was, that 24 hours after incubation the outflow medium from the EVEIT chamber showed a concentration of at least 2 mmol/L glucose and more than 1.5 mmol/L lactate (measured with GOD-PAP or LOD-PAP; Greiner Diagnostic GmbH, Bahlingen, Germany). Furthermore, corneas were stained with sodium fluorescein and illuminated with cobalt blue light to detect epithelia defects. Only corneas that were fluorescein negative at the time of quality control were included. To complete the initial quality control all corneas were examined for complete transparency using fluoroscopy on the examination loupe. The vital and fully epithelialized corneas could then be cultured under stable hydrostatic pressure (6 cm water column) with MEM at 32°C and 100% humidity. This guaranteed an optimal culture condition for several days. This procedure has already been published [14].
EVEIT Corneas in the Deficiency Culture to Produce Corneal Edema
To simulate a corneal edema the culture medium was replaced with a hypoosmolare culture medium after quality control. The hypoosmolare medium consists of a mix of the standard medium, MEM, with 0.3% saline solution in a ratio of 1:4, resulting in an osmolality of 148 mOsm/kg. The corneas cultured in the EVEIT system were then cultured in an incubator at 32°C and 100% humidity [15]. This resulted in a homogenous increase in the thickness of all corneas. Daily vitality testing of the corneas using glucose-lactate quantification and fluorescein staining was performed as described above.
Osmolality of the test Substances Used
We prepared suspensions of the following substances: Bee Honey (Honig Flotte Biene, Langnese Honig GmbH & Co. KG®, Hammoorer Weg 25, Bargteheide); Powder sugar (Pfeiffer Langen GmbH & Co. KG®, Aachener Str. 1042a, Köln, Germany); Substance C188 (BASF®, Carl-Bosch-Str. 38, Ludwigshafen, Germany); Prevor® Substance X (Prevor® Von-Werth-StraΒe 37, Köln, Germany). The experimental and theoretical osmolality of the different substances was determined via dilution series in distilled water and mathematical extrapolation. For this purpose, the substances were measured in a dilution series with Aqua-bidest using the crystallisation depression point method in the Gonotec® Osmomat (Osmomat 3000, Gonotec GmbH, Berlin). From these data, the osmotic effectiveness of the individual substances was extrapolated by means of linear correlation analysis for fully dissociated substances: substance X, icing sugar. A polynomial correlation analysis was used for extrapolation for micelle formers or not fully dissociated substances. By means of the mixing ratios in the suspensions, balanced osmo-capacities were attempted to be achieved. Since the therapeutics tested here do not mix with the oily amphiphilic suspension base, the substance (Sovermol) DOL (BASF® Carl-Bosch-Str. 38, Ludwigshafen, Germany), suspensions were prepared. The unpreserved ODM5® solution from TRB-Chemedica was used as reference substance.
From the resulting functional equations (Figure 1), theoretical osmolalities (tOsmol) of the individual substances were determined at a defined concentration of 1g/1g substance. This results in an osmolality of the individual substances (1-n) in the suspension in their weight fractions [g] as the sum divided by the total weight [g(total)] (amphiphilic solvent + osmotically active substances). As a calculation example for the entries in Table 1 for S1, the formula is given here:
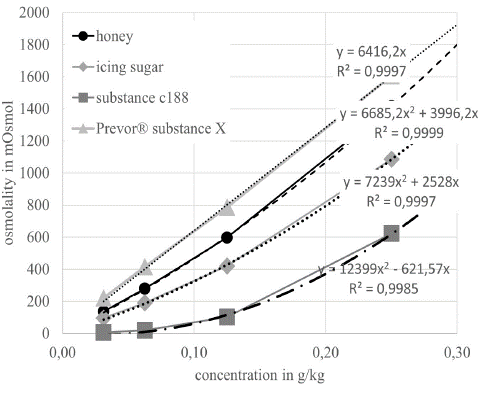
Figure 1: shows the osmolality of the different substances with increasing concentration in Aqua-bidest.
Calculated Osmolality of Suspension S1, Table 1:
(tOsmol (honey)*2.5g + tOsmol (substance C188) * 0.2g) / g(total) = (10,681.4 mOsmol * 2.5g + 11,777.4 mOsmol * 0.2g) / 12.7g = 2,2881 mOsmol
Results
All corneas showed the swelling of the corneas as described by Dutescu et al. In this study, the corneas swelled from an average original thickness of 429 μm (+/- 29 μm) (n=24) to a thickness of 563 μm (+/- 28 μm) (n=24) after 20 hours in a hypoosmolare deficiency medium (minute -50 to 0 in (Figures 2, 3 and 6).
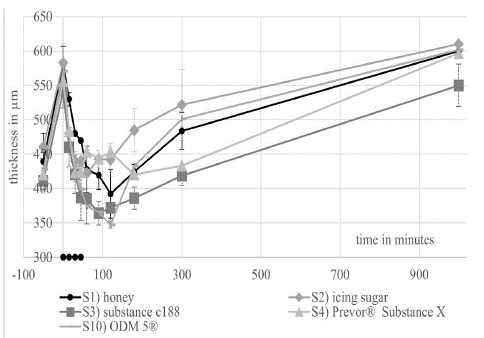
Figure 2: shows the course of the thickness during the short-term experiment. The value -50 minutes corresponds to the time before the addition of the deficiency medium.
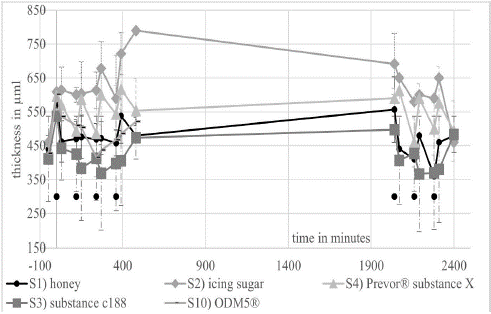
Figure 3: shows the thickness of the corneas in the long-term experiments over 2 days. The initial thickness of the corneas was determined at time point -50.
EVEIT Short-Term Experiments
The EVEIT short-term experiments showed a generally efficient deswelling effect of all substances (Figure 2). S1) honey, S3) substance C188 and S10) ODM5® caused a decrease in thickness to corneal thickness below the initial thickness of the EVEIT corneas. The most successful was the use of substance No. 10) ODM5® and S3) substance C188. The least pronounced was the deswelling caused by S2) icing sugar. After 90 minutes, a return of corneal edema was observed, with the thickness increasing again to 585 μm (+/- 31 μm), after 14 hours (rebound effect).
EVEIT Long-Term Experiments
The EVEIT long-term experiments showed different efficacy profiles of the substances. S1) Honey showed a quite effective deswelling of the corneas over both days. As shown in Figure 5, there was a decrease in corneal edema of almost 48% (see discussion). S2) icing sugar caused an exceptionally large increase in corneal edema in some cases and also caused epithelial damage seen in (Figure 4). Figure 4 shows that, on average, icing sugar was less effective at deswelling than the non-suspended pure DOL. Deswelling was particularly strong with S3) substance C188, which brought the corneas below the initial thickness of the corneas and maintained this deswelling throughout the day. In addition, the overnight rebound effect was less pronounced in these corneas. With S4) Prevor® substance X, there are strong fluctuations in corneal thickness. The coating of the corneas does not seem to be sufficient to allow effective prolonged deswelling beyond 2 hours. In addition, the epithelial damage shown in Figure 4 occurs. S2) and S4) only remove about as much of the edema as pure DOL over the entire period (Figure 5). The use of S10) ODM5® was successful in treating the model of epithelium-sparing corneal deswelling used here.
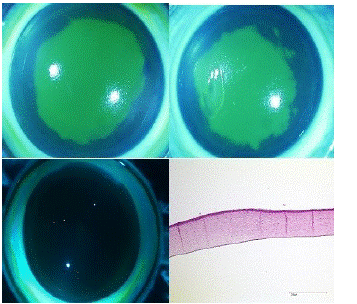
Figure 4: shows on the top left a rabbit cornea after 3-day coating with S2) icing sugar. This was photographed here under cobalt blue light after fluorescein staining. At the top right we see a rabbit cornea after 3-day coating with S4) Prevor® substance X. Both show clear epithelial damage. In the lower left picture, we see a cornea after 3-day coating with ODM5® under cobalt blue light after fluorescein staining with intact epithelium. Below right is the histological section of an EVEIT cornea, in haematoxylin-eosin staining, after 3-day treatment with DOL with intact epithelium and endothelium with a light focal edema.
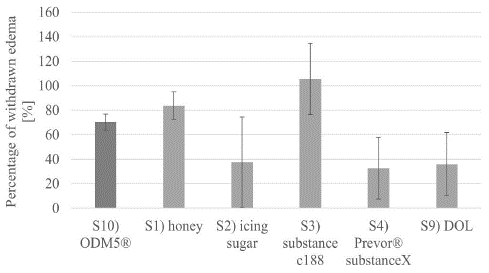
Figure 5: shows the decrease in corneal thickness averaged over the day/total amount of corneal thickness increased.
Further Mixtures in Long-Term Experiments
Based on these results, we conducted further EVEIT experiments with significantly lower concentrations of substance C188 in DOL, namely S5–7). We also tested the effect of a suspension of substance C188 which had previously been pre-swollen with distilled water in a defined manner (S8). Figure 6 shows an effective deswelling over the day with the tested substances. Together with the concentration of substance C 188 the deswelling effect of the suspensions tested decreases. The addition of distilled water to the suspension of substance C188 S8 showed an improvement in the deswelling properties of the suspension, although the theoretical osmolality of 1845 mOsmol for S6 drops to 1039 mOsmol for S9 due to the addition of water.We also saw swelling effects in corneas treated with DOL (S9), the carrier substance. At the same time we observed a deswelling effect on some parts of the same corneas. This suggests that unforeseen influences, for example evaporation, had an additional effect during the experiment [16]. The corneas treated with pure DOL S9) showed a very irregular surface in the histological examination (Figure 4) as well as in the OCT. These irregularities were also seen in many other corneas treated with substances S1–8.
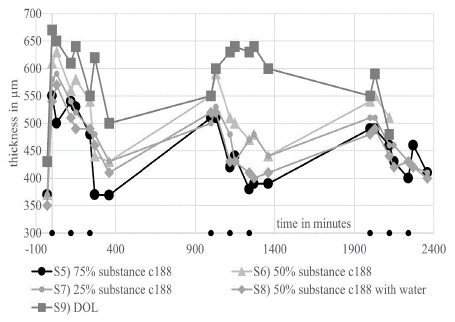
Figure 6: Corneal thickness curve of the EVEIT corneas in the long-term experiment with the mixtures S5–8 and S12.
Glucose Lactate Test
Figure 7 shows a clear decrease in the glucose and lactate concentrations after the hypoosmolare medium was added, due to the corresponding dilution of this medium. Only a slight increase in lactate is noticeable in the corneas treated with honey.
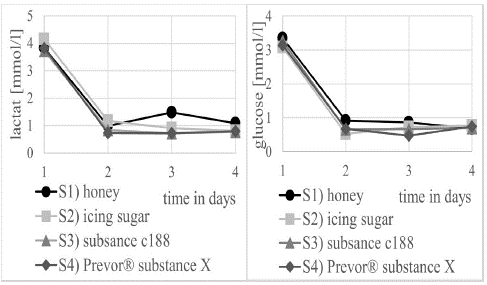
Figure 7: Concentration curve of lactate (left) and glucose (right) in the medium from the artificial anterior chamber over the experimental days during treatment with S1 honey, S2) icing sugar, S3) substance C188 and S4 Prevor® substance X.
Discussion
In the following, the deswelling effect of the therapeutics on corneal edema will be discussed with special focus on their osmotic properties and on the influence of the measuring methods. All rabbit corneas examined here experienced an average increase in thickness of 133.6 μm during the swelling phase after addition of the deficiency medium. With a corneal diameter of approximately 12 mm [17], this results in an increase in corneal volume of approximately 0.025 ml. To move this additional amount of water out of the cornea, the amount of osmotically active particles, on the other side of the semipermeable membrane of the corneal epithelium, that dissolve in this 0.025 ml must be high enough to reach an osmolality of approximately 320 mOsmol/L, equal to the osmolality of a healthy cornea [18]. Without considering the osmolarity of the tear film or other influencing variables, an amount of 0.0105 mOsmol must be applied to the semipermeable membrane of the corneal epithelium to remove the water from the cornea. According to the calculations in (Table 1), we have safely achieved these quantities in all experimental groups. However, the contact to the corneal surface due to internal convection or mixing of the suspension is decisive for the deswelling effect. In absence of eyelid blink, this is only incompletely represented in the EVEIT model. The sedimentation speed of suspensions cannot be used as a model because of the thin layer thickness of the applied suspensions. An artificial blink was not yet available in the EVEIT model at that time.
Reference
Ingredients
Osmolality used per gram of suspension in mOsmol
N=
S1
2.5 g honey with 0.2 g substance C188 (as emulsifier) in 10 g DOL
2.2881
4
S2
2.5 g icing sugar in 7.5 g DOL
2.442
3
S3
3 g substance C® 188 in 14 g DOL
2.078
5
S4
0.85 g Prevor® substance X, 2.15 g substance C 188 in 14 g DOL
321
3
S5
1.3 g substance C188 in 7g DOL
1.845
1
S6
0.75 g substance C188 in 7g DOL
1140
1
S7
0.375 g substance C188 in 7g DOL
599
1
S8
0.75 g substance C188 in 0.75 g distilled water. In 7 g DOL
1039
1
S9
Pure DOL
0
2
S10
ODM5®
2
table 1
Table 1: Suspension compositions in [g] of the respective substances.
Measuring Technique
In the first experiments, the thickness was measured using ultrasound pachymetry. It was advantageous that the corneas could remain in the incubator and did not dry out due to significantly lower temperature and humidity [19]. The disadvantage, however, was that this measurement did not work reliably and the epithelium, which was under osmotic stress, developed undesirable epithelial defects due to the contact of the probe with the cornea. For this reason, we continued the experiments using noncontact measurements, particularly the OCT. This way we avoided epithelial defects caused by the measurement method and were able to make more reliable statements about the epithelial damage caused by the tested substances. However, due to the OCT measurement, a certain drying of the corneas is a source of error that should not be neglected. To examine the corneas on the OCT, they must be taken out of the incubator and examined at room temperature for about 2 minutes. This disturbance was particularly evident in the corneas treated with S9), the pure DOL. Some of them became thinner (Figure 6), although DOL has no osmotic properties. In the article on perspiratio insensibilis [19] we were able to show that this was probably due to long contact with the cold and dry laboratory air. Unfortunately, this could not be avoided due to the measurement method (OCT). Since, according to this finding, the error is the same for all corneas due to uniformly short processing times, a systematic falsification of the results can be safely ruled out.
Model Discussion: Suitability
The model used here is suitable for simulating corneal edema because it allows a constant influx of water into the cornea while it is constantly nourished [20]. In preliminary experiments, full reversibility had already been shown when the original culture conditions were restored [15]. The homogeneity of suspensions and thus the fineness of distribution is a pharmaceutical-technical problem that we did not have fully under control. Homogenisation steps with mechanical stirring, ultrasonic bath and slight heating produced optically homogeneous suspensions. However, the homogeneity could not be measured with the methods available to us. We interpret focal differences in thickness, which we saw in some corneas, as caused by such in homogeneities in the suspensions.
EVEIT Short-Term Experiments
The short-term experiments showed quite impressively that the therapeutics we used were capable of moving sufficient water from the cornea across the epithelium so that the swollen corneas became thinner than baseline in some cases. It was also clear that the mixture in which we used Substance C188 in S3) was comparable to the effect of ODM5®. It was particuThe short-term experiments showed quite impressively that the therapeutics we used were capable of moving sufficient water from the cornea across the epithelium so that the swollen corneas became thinner than baseline in some cases. It was also clear that the mixture in which we used Substance C188 in S3) was comparable to the effect of ODM5®. It was particularly striking that, unlike in the study by Zander et al. (2021) [9] where a decrease in corneal thickness by ODM5® after two drops led to a decrease in corneal thickness of only -10.5 μm, ODM5® in our study led to a decrease of 196 μm after one hour. Admittedly, we used 4 drops, and in the EVEIT model, as described above, no blink could be simulated, as shown in the study of Zander et al. This naturally leads to significantly longer exposure times. We would therefore attribute the decrease in thickness described by Zander et al. less to ODM5® than to perspiratio insensibilis.
EVEIT Long-Term Experiments
Although S1) honey showed a good deswelling effect, it also damaged the corneal epithelium due to the composition used here, which is why we are neglecting honey from the further planning of the tests for the time being. The S2) approach with icing sugar had a partial de-swelling effect but after some time even a swelling effect on the EVEIT corneas tested here. We assume that this could be due to the penetration of the glucose into the cornea. In case of the S3) Substance C188 approach, an overdose of the osmotic agent can be suspected, as the corneas also shrank below the initial thickness before swelling over most of the day. This can also explain small epithelial defects. Overall, at lower concentrations of substance C188, there is significantly less corneal damage. At the concentration of S4) used here, the deswelling effect does not seem to be sufficiently dosed by the individual components to achieve proper deswelling. In particular, the deswelling was not sustainable.
Potential Increase in Osmolality
Concerning the osmolalities of the tested substances in aqua-bidest the osmolality of especially substance C188, honey and perhaps also icing sugar does not increase linearly with rising amounts of water (Figure 1). This observation is also found in Busmann et al. (2020) [21] for substance C188 HS15 where a plateau phase of the osmolality is described. Similar observations have been made for other surfactant molecules. By heating, these molecules can be converted into a gel state, whereby this property is lost (Tacey et al. 1998) [22]. Biochemically, it is known that strongly hygroscopic polymers show steric inhibition of their water uptake during the transition from the dry to the wet phase. This also explains stronger hydration of the osmotically active molecules of the substance C188 at higher concentrations of water.
Epithelial Damage due to Osmotic Stress
In an in vitro study, it was shown that cultured corneas tolerate osmolarities of up to 350 mOsm/L before cytotoxic effects occur (Kang et al. 2014) [23]. However, since eye drops are rapidly removed from the surface, it is difficult to establish exact limits for these toxic thresholds. In clinical studies, patients tolerate the 6-fold application of eye drops with an osmolarity of 380 mOsm/L without toxic effects. Patients with Dry Eye Syndrome (DES) who wear hypertonic contact lenses also show no toxic changes (Thulasi et al. 2017) [24]. Patients also report preferential use of 280 mOsm/L eye drops as they were found to be less uncomfortable [25]. The use of highly hypertonic ophthalmic rinses after eye burns also does not lead to eye damage [26]. The frequent application of unpreserved iso- and hyperosmolar eye drops leads to surface changes of the epithelia in the expression of the microvilli [27]. The long-term use of hyperosmolar aqueous eye drops in the context of Fuchs' endothelial dystrophy with ODM5®, Ocusaline® and Omnisorb®are clinically common. The low ocular irritation with these eye drops, which are usually between 1200 and 1400 [28] to 1900 [15] mOsmol/kg, can be explained by tearing and blinking and the constant production of tear fluid, which removes the hyperosmolar eye drop from the ocular surface relatively quickly. Since neither blinking nor tear production are present in the EVEIT, this appears to be a cause for the considerable epithelial damage caused by strongly de-swelling substances in the long-term EVEIT experiment. However, the clinically used substance ODM5® did not show any epithelial damage in our trials. It was particularly striking that a mixture of substance C188 with water and then with DOL had a positive effect on the deswelling of the corneas. Overall, it can be shown that the medicinal deswelling of the corneas based on an amphiphilic carrier such as DOL is promising, and further studies based on these results are necessary to develop appropriate therapeutics.
References
- Maurice DM. The structure and transparency of the cornea. J Physiol. 1957; 136: 263-86.
- Sharma N, Singhal D, Nair SP, Sahay P, Sreeshankar SS, et al. Corneal edema after phacoemulsification. Indian J Ophthalmol. 2017; 65: 1381-1389.
- SIMPSON GV. Corneal edema. Trans Am Ophthalmol Soc. 1949; 47: 692-737.
- Medicinal eye therapy; Paul U. Fechner, Klaus D Teichmann, 4th edition; 2000 Georg Thieme Verlag, Stuttgart New York p. 359
- Zander DB, Böhringer D, Fritz M, Grewing V, Maier PC, et al. Hyperosmolar Eye Drops for Diurnal Corneal Edema in Fuchs’ Endothelial Dystrophy: A Double-Masked, Randomized Controlled Trial. Ophthalmology. 2021; 128: 1527-1533.
- Insler MS, Benefield DW, Ross EV. Topical hyperosmolar solutions in the reduction of corneal edema. CLAO J. 1987; 13: 149-51.
- Levenson JE. Corneal edema: cause and treatment. Surv Ophthalmol. 1975; 20: 190-204.
- Costagliola C, Romano V, Forbice E, Angi M, Pascotto A, et al. Corneal oedema and its medical treatment. Clin Exp Optom. 2013; 96: 529-35.
- Zander DB, Böhringer D, Fritz M, Grewing V, Maier PC, et al. Hyperosmolar Eye Drops for Diurnal Corneal Edema in Fuchs’ Endothelial Dystrophy: A Double-Masked, Randomized Controlled Trial. Ophthalmology. 2021; 128: 1527-1533.
- Reim M, Hesse R, Pietruschka G. The metabolism of organ cultures of cornea in TC 199 with added dextran 500 or hydroxyethyl starch 450. Clin Monbl Ophthalmology. 1990; 196: 76-80.
- Kaufman HE, Beuerman RW, Steinemann TL, Thompson HW, Varnell ED. Optisol corneal storage medium. Arch Ophthalmol. 1991; 109: 864-8.
- Ljungström KG, Renck H, Hedin H, Richter W, Wiholm BE. Hapten inhibition and dextran anaphylaxis. Anaesthesia. 1988; 43: 729-32.
- Chiaradia PA, Zeman Bardeci LA, Dankert S, Mendaro MO, Grzybowski A. Hot Topics in Dry Eye Disease. Curr Pharm Des. 2017; 23: 608-623.
- Frentz M, Goss M, Reim M, Schrage NF. Repeated exposure to benzalkonium chloride in the Ex Vivo Eye Irritation Test (EVEIT): observation of isolated corneal damage and healing. Altern Lab Anim. 2008; 36: 25-32.
- Dutescu RM, Panfil C, Schrage N. Osmolarity of prevalent eye drops, side effects, and therapeutic approaches. Cornea. 2015; 34: 560-6.
- Schrage T, Panfil C, Schrage N, Fuest M, Urbach M. perspiratio insensibilis of the cornea in the EVEIT system. Graefes Archives Ophth. In press 2022.
- Doughty MJ. The cornea and corneal endothelium in the aged rabbit. Optom Vis Sci. 1994; 71: 809-18.
- Kompa S, Schareck B, Tympner J, Wüstemeyer H, Schrage NF. Comparison of emergency eye-wash products in burned porcine eyes. Graefes Arch Clin Exp Ophthalmol. 2002; 240: 308-13.
- Schrage T, Panfil C, Fuest M, Schrage N, Urbach M. PerspiratioInsensibilis of the Cornea. J Ophthalmol & Vis Sci. 2023; 8: 1072.
- Ralf M. Dutescu, MD, Claudia Panfil, Prof. Norbert Schrage. Comparison of the effects of various lubricant eye drops on the in vitro rabbit corneal healing and toxicity. Experimental and Toxicologic Pathology. 2017; 69: 123-129.
- Busmann EF, Martínez DG, Lucas H, Mäder K. Phase inversion-based nanoemulsions of medium chain triglyceride as potential drug delivery system for parenteral applications. Beilstein J Nanotechnol. 2020; 11: 213-224.
- Tacey X Viegas, Raymond L Henry. Osmoticbehavior of poloxamer 407 and other non-ionic surfactants in aqueous solutions. International Journal of Pharmaceutics. 1998; 160: 157-162.
- Kang SS, Ha SJ, Kim ES, Shin JA, Kim JY, et al. Effect of nerve growth factor on the in vitro induction of apoptosis of human conjunctival epithelial cells by hyperosmolar stress. Invest Ophthalmol Vis Sci. 2014; 55: 535-541.
- Thulasi P, Djalilian AR. Update in Current Diagnostics and Therapeutics of Dry Eye Disease. Ophthalmology. 2017; 124: S27-S33.
- Fletcher E, Brennan NA. The effect of solution tonicity on the eye. Clin Exp Optom. 1993; 76: 17-21.
- Wiesner N, Dutescu RM, Uthoff D, Kottek A, Reim M, Schrage N. First aid therapy for corrosive chemical eye burns: results of a 30-year longitudinal study with two different decontamination concepts. Graefes Arch Clin Exp Ophthalmol. 2019; 257: 1795-1803.
- Schrage N, Wuestemeyer H, Langefeld S. Do different osmolar solutions change the epithelial surface of the healthy rabbit cornea? Graefes Arch Clin Exp Ophthalmol. 2004; 242: 668-73.
- Zander DB, Böhringer D, Fritz M, Grewing V, Maier PC, et al. Hyperosmolar Eye Drops for Diurnal Corneal Edema in Fuchs’ Endothelial Dystrophy: A Double-Masked, Randomized Controlled Trial. Ophthalmology. 2021; 128: 1527-1533.