
Research Article
J Ophthalmol & Vis Sci. 2023; 8(3): 1086.
Full Thickness Macular Hole after Laser Assisted in Situ Keratomileusis: A Case Report and Review of the Literature
Sverdlichenko I¹; Tayeb S²; You Y3,4; Cruz-Pimentel M²; Yan P2,5,6*
1Faculty of Medicine, University of Toronto, Toronto ON, Canada
2Department of Ophthalmology and Vision Sciences, University of Toronto, Toronto ON, Canada
3Save Sight Institute, University of Sydney, Sydney, Australia
4Macquarie Medical School, Macquarie University, Australia
5Donald K. Johnson Eye Institute, University Health Network, Toronto ON, Canada
6Kensington Vision and Research Center, Toronto ON, Canada
*Corresponding author: Peng Yan Department of Ophthalmology and Vision Sciences, University of Toronto 27 King’s College Cir, Toronto, ON, Canada Tel: 416-928-1335; Fax: 416-928-5075 Email: pyan@kensingtonhealth.org
Received: August 22, 2023 Accepted: October 03, 2023 Published: October 10, 2023
Abstract
Purpose: While myopia is known to be a risk factor for Macular Hole (MH) formation, there have been no reviews evaluating the association between LASIK procedure and MH development. Our case series and literature review examine the clinical characteristics and visual outcomes of patients who developed MH following LASIK.
Methods: A retrospective chart review was completed for two patients who developed MH following LASIK. For the literature review, Ovid/MEDLINE was searched for all patients who developed MH following LASIK.
Results: Two patients with history of LASIK procedure presented with unilateral MH. Both patients were only mildly myopic and lacked other risk factors for MH. They both underwent pars plana vitrectomy, Internal Limiting Membrane (ILM) peel with ILM flap draped over and tucked into the macular hole, followed by 20% sulfur hexafluoride tamponade. Both cases had MH closure and improvement in final visual acuity. The literature review included 25 cases and 27 eyes with MH following LASIK. Twenty-four cases were female and myopic, and the mean refraction was -8.64 D. The average duration from LASIK to MH development was 15.3 months. Mean preoperative best corrected visual acuity was Snellen 20/235. Seventy-seven percent (21/27) of eyes underwent vitrectomy, with a MH closure rate of 100% and final visual acuity of Snellen 20/89.
Conclusion: Myopia is a known risk factor for MH formation. However, LASIK procedure may introduce an additional increased risk of MH formation in myopic eyes. Thus, although rarely reported, patients considering LASIK should be counselled on this potential risk.
Keywords: Macular hole; Laser assisted in situ keratomileusis; LASIK; Vitrectomy
Abbreviations: MH: Macular Hole; LASIK: Laser Assisted In Situ Keratomileusis; OD: Right Eye; OS: Left Eye; OCT: Optical Coherence Tomography; BCVA: Best Corrected Visual Acuity; SF6: Sulfur Hexafluoride; ILM: Internal Limiting Membrane; PPV: Pars Plana Vitrectomy; PVD: Posterior Vitreous Detachment
Introduction
Macular Hole (MH) is a vitreoretinal interface disease characterized by a full-thickness neurosensory retinal defect in the center of the macula [1]. There are two types of MHs: idiopathic MH, which is caused by the tangential tractional force that may be exerted by pre-existing epiretinal membrane, or anteroposterior by vitreomacular traction on the fovea; and traumatic MH, usually caused by mechanical blunt injury of the eye. The incidence of developing an idiopathic MH has been reported as 0.02% in some studies, with 80% being unilateral and occurring at a mean age of 62.6 years [2]. Females have a 64% increased risk of developing MH compared to males, after adjusting for confounding factors [2]. MH is also a common complication in pathological myopic eyes with an axial length greater than 26.5mm and/or refraction greater than -6.00 diopters [3].
Laser-Assisted in Situ Ketatomileusis (LASIK) is a common ophthalmologic procedure that has been used for the correction of low to moderate myopia [4]. Vitreoretinal complications including endophthalmitis, retinal tearing and detachment, retinal hemorrhage, and choroidal neovascular membrane have been reported. Various case reports and case series have also identified MH formation occurring following LASIK, in both myopic and non-myopic eyes [5-10]. The potential mechanism for this is likely sudden increase and decrease in the compressional pressure exerted to the vitreous and vitreomacular interface by the suction cup that is placed over the eye during LASIK procedure.
In this study, we report on 2 young patients who developed MH following LASIK procedure for the correction of myopia and an internal limiting membrane tucking technique in the management of MH. Additionally, we provide an overview of literature-documented cases of MH developing after LASIK, describe their presentation, surgical management, and outcomes. We discuss the proposed pathogenesis of MH formation following LASIK procedure.
Materials and Methods
A chart review was completed of two patients who developed MH after LASIK procedure. We recorded data on age, sex, time interval between LASIK procedure and MH formation, ophthalmologic exam findings including dilated fundus exam, Optical Coherence Tomography (OCT) and Best Corrected Visual Acuity (BCVA). We report on surgical procedures and outcomes of these patients. Institutional research ethics board approval was received from the University of Toronto. Informed consent was obtained from the patients for use of their clinical data. This study adheres to the Tenets of the Declaration of Helsinki.
For the literature review aspect of this study, we analysed published literature of patients who developed MH after LASIK procedure. Ovid/MEDLINE was searched for all literature containing the key terms macular hole and Laser in Situ Keratomileusis, LASIK. The requirements for inclusion were that the paper was written or translated into English, the patient(s) had a confirmed diagnosis of MH on ophthalmologic exam, and BCVA at time of MH diagnosis and at final follow-up were reported.
Case Report
Case 1
A 38-year-old female was referred to retina service with a four-day history of decreased vision due to newly identified MH. Her ocular history was unremarkable except for bilateral LASIK procedure. The myopia was mild, and she had no other risk factors, including no history of prior trauma or other ocular surgery other than LASIK. OCT confirmed a full-thickness MH in the right eye (OD) with intact posterior hyaloid (Figure 1). Visual acuity was Snellen 20/100 OD. The patient underwent uncomplicated Pars Plana Vitrectomy (PPV), Internal Limiting Membrane (ILM) peel with temporally hinged inverted ILM flap draped over the MH and gently tucked into the macular hole using the tip of the ILM forceps followed by 20% sulfur hexafluoride (SF6) tamponade. Visual acuity achieved Snellen 20/60 OD at two weeks postop. One month postoperatively, OCT confirmed the successful anatomical closure of the MH. Five months later at her last follow-up appointment, visual acuity improved to Snellen 20/30 OD with correction.
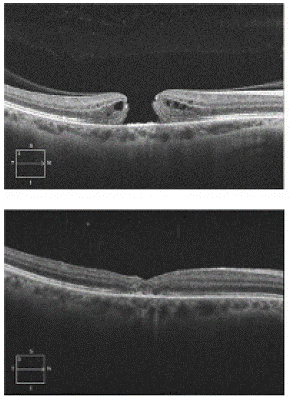
Figure 1: Top panel shows OCT image demonstrating full-thickness macular hole in the right eye with intact posterior hyaloid. Bottom panel shows post-operative OCT image taken one month after presentation with successful macular hole closure.
Case 2
The second case was a 47-year-old female who had a history of LASIK procedure OU for mild myopia, as well as a lamellar defect confirmed on OCT in the right eye. There were no other risk factors for this patient. OCT confirmed a lamellar defect OD as well as a small full thickness MH OD (Figure 2). The BCVA at that time was Snellen 20/60+2 OD. The patient underwent uncomplicated PPV, ILM peel to achieve a temporally hinged ILM inverted flap that was draped over the macular hole and gently tucked into the macular hole using the tip of the ILM forceps followed by 20% SF6 tamponade.
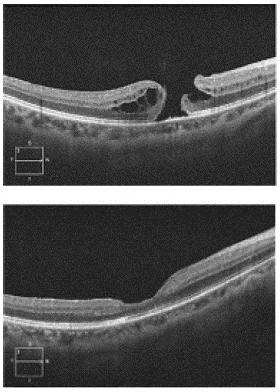
Figure 2: Top panel shows OCT image demonstrating lamellar defect and small full thickness macular hole in the right eye. Bottom panel shows post-operative OCT image taken six months after first presentation, demonstrating macular hole closure.
On post-operative day one, the retina was attached with ILM draped over the MH. By one week post-operatively, the MH was confirmed to be closed on OCT. One month post-operatively, the BCVA was Snellen 20/30. A nuclear sclerotic cataract was noted in both eyes, which was observed. By 6 months, the vision was stable at Snellen 20/40-2 OD.
Literature Review
The literature review identified 6 studies reporting on 25 patients and 27 eyes with MH formation following LASIK procedure (see Table 1 for overview of studies). Ninety-six percent (24/25) of the cases were female, with a mean age of 44.5 years (SD: 11.3; range 21 – 65 years). Additionally, 96% (24/25) of cases were myopic, with a mean refraction of -8.64 D (SD: 4.0, range: -0.5 D to -19.75 D). Most of the patients had unilateral MHs (92%, 23/25), and the average number of months between LASIK procedure and diagnosis of MH was 15.3 months (SD: 28.8, range 1 – 83 months). More than half (14/26) of the MHs were Stage 4 (full-thickness MH with complete separation of the vitreous from the macula and optic disc). The mean BCVA at the time of MH diagnosis was Snellen 20/235 (Range: 20/40 to light perception, logMAR 1.07, SD: 0.66). Seventy-seven percent (21/27) of the eyes underwent PPV to close the MH. ILM peeling was also done in 7 of the 21 eyes. Use of intraocular gas tamponade was reported in 59.3% (16/27) of eyes, and for three eyes it was specified to be SF6. In two cases, silicone oil was used. The MH closed in all 21 eyes that underwent PPV, while outcomes were not reported in 6 eyes that did not have surgery. The final mean BCVA was Snellen 20/89 (Range: 20/20 to counting fingers, log MAR 0.65 SD: 0.58).
Study
N, Sex
Age
Uniliteral or Bilateral MH
Myopic (Y/N)
Refractive Spherical Equivalent
Months from LASIK to MH
Ocular Findings
BCVA of affected eye(s) at MH Diagnosis
Surgical Technique
Gas Used
Follow-up Time (months)
MH Closure Y/N
Final BCVA
Harasawa et al. 2014(5)
F
21
Unilateral (RE)
N
N/A
7
Stage 3 macular hole (full-thickness macular hole) in RE
20/200 (RE) (logMAR 1.0)
23-guage PPV with internal limiting membrane peeling
SF6 12% tamponade
2
Y
Snellen 20/20 (logMAR 0)
Bikbova et al. 2013(6)
F
26
Unilateral (LE)
Y
LE: -7.25 D
2
Stage 4 macular hole in LE (full-thickness macular hole)
0.5 (LE) (logMAR 0.3)
PPV with internal limiting membrane peeling
Intraocular gas tamponade (not specified)
3
Y
BCVA LE 0.7 (logMAR 0.16)
Garcia-Fernandez et al. 2012 (26)
F
53
Bilateral
Y
RE: -8.0 D
LE: -8.0 D132
Stage 4 full-thickness macular hole in both eyes with subretinal fluid surrounding the defect, and absence of yellow deposits on RPE. Also, PVR in both eyes
0.4 (RE) (logMAR 0.4)
0.2 (LE) (logMAR 0.7)23-guage PPV
SF6
6
Y
BCVA RE 1.0, logMAR 0
BCVA LE 0.6, logMAR 0.22Arevalo et al. 2005
(8)19, 94.7% (18) F
M: 46 years (range 25-65)
92.3% (18/19) Unilateral
100% Myopic
Range: -0.50 D to -19.75 D
Mean: -8.9 DM: 12.1 months (range 1-83)
60% (12/20) eyes stage 4 MH
10% (2/20) eyes with epiretinal membrane
80% (16/20) eyes had subretinal fluid surrounding MH
70% (14/20) had 20/200 or worse vision (logMAR 1.0)
PPV + gas: 40% (8/20)
PPV + gas + ILM: 15% (3/20)
PPV + ILM + silicone oil: 5% (1/20)
PPV + gas + laser: 5% (1/20)
PPV + silicone oil: 5% (1/20)
None: 30% (6/20)
Gas: 85.7% (12/14)
Silicone oil: 14.3% (2/14)
1-70
MH closed in all 14 patients who underwent PPV (outcome of 6 other patients not reported)
45% (9/20) had 20/200 or worse vision (logMAR 1.0)
Chan et al. 2001
(10)F
F
48
36
Unilateral (RE)
Unilateral (RE)Y
Y
RE: -6.9 D
RE: -8.5 D
1.75
2
Stage 2 MH RE
Stage 2 macular microhole RE
20/50 (logMAR 0.4)
20/70 (logMAR 0.54)
PPV
PPV
Not reported
Not reported
3
5
Y
Y
20/25 (logMAR 0.1)
20/30 (logMAR 0.18)Ruiz et al. 2002(8)
F
53
Unilateral (LE)
Y
LE: -6.75 D
12
MH with small yellow deposits on RPE
20/100 (logMAR 0.7)
PPV with internal limiting membrane peeling
Could not be obtained
Could not be obtained
Yes
20/50 (logMAR 0.4)
Table 1: Overview of studies included in literature review.
Discussion
In this study, we report on two young female patients diagnosed with MH. Both patients had LASIK procedure for correction of myopia with no other risk factors. In both cases, given young age with no history of trauma, the posterior hyaloid was intact, myopia was mild, and the patients had no other risk factors for MH formation including no Posterior vitreous detachment (PVD), and no history of ocular trauma. The only potential risk factor for MH formation was the patients’ mild myopia and prior LASIK procedure. Following PPV surgery with ILM flap draped and tucked into the MH, the postoperative BCVA improved to Snellen 20/30 or 20/40 in both eyes. In the literature review, we report on 25 patients that developed MH after LASIK procedure. Ninety-six percent of the cases were female and myopic, with an average refraction of -8.64 D. All but two of the cases had unilateral MHs, and the time from LASIK procedure to formation of MH was quite variable, but on average just over one year. Three quarters of the eyes underwent vitrectomy and of those 33% underwent ILM peel to close the MH, and there was an overall improvement in BCVA from Snellen 20/235 (logMAR 1.07) to 20/89 (logMAR 0.65).
It was interesting to note that almost all patients in the review and both patients in our case report were female. One hypothesis for this finding may be that females are more likely to undergo LASIK for cosmetic purposes, hence a potential bias, which may have influenced the outcomes in the review. Another possibility is that myopia is a known risk factor for MH development, and almost all eyes in the study were myopic. Previous studies have reported higher incidence of myopia, and greater myopia progression and axial elongation in females [11]. When reviewing the treatment of MH, most cases in the literature were surgically managed with PPV to close the MH, and only one third of those cases had ILM peel, with details of the ILM peeling technique not described in the studies. Additionally, 60% had intraocular gas tamponade with or without ILM peeling. Our cases were managed with PPV, ILM peeling with ILM flap draped over and gently tucked into the MH followed by 20% SF6 gas tamponade. There has been debate around whether the inverted ILM flap technique can achieve better anatomic outcomes than the conventional ILM peeling technique, with studies having mixed results [12-14]. In our literature review and case report, there was 100% closure rate in all reported cases, although we are unable to rule out report bias.
The pathogenesis of idiopathic MHs remains controversial. Vitreofoveal traction is felt to be the predominant force, together with pre-existing risk factors. LASIK may have characteristics that induce this tractional force. Ruiz-Moreno reported an incidence of 0.01% (1/8972) of MH developing after LASIK procedure [15]. Arevalo postulated that the sudden increase and decrease in intraocular pressure (IOP) to levels over 60 mmHg may cause acute vitreoretinal traction at the vitreous base and posterior pole [16]. They hypothesized that when the suction ring is in place, the eye deforms along the anterior-posterior axis and the diameter of the globe may increase. At the same time, because the eye is a closed system, it must contract along the horizontal axis and the equatorial diameter may decrease. When the suction stops and the suction ring is released, decompression leads to a dynamic overshoot with equatorial elongation and anterior-posterior contraction. All these may cause acute vitreoretinal traction at the vitreous base and posterior pole. Mirshahi further hypothesized that another contributing factor is the suction ring itself, which creates a considerable ring-shaped suction at the paracorneal parts of the sclera [17]. This traction power might affect the vitreous base, resulting in PVD which may contribute to MH formation. Arevalo also postulated that the excimer-laser-induced shock wave may play a role in the development of PVD. However, Krueger et al. evaluated stress wave amplitudes during laser surgery of the cornea, and suggested that they are harmless for posterior retinal and subretinal structures [18]. Lastly, the rapid increase of IOP to 60mmHg with release can cause antero-posterior traction and sudden changes to the vitreous, potentially leading to MH formation. Thus, despite many proposed mechanisms there remains controversy about the causal relationship of LASIK procedure and MH formation, and further study of this topic is warranted.
It is important to recognize that myopia is itself an independent risk factor for MH formation and potential cause of macular hole formation in our cases. For myopic eyes, factors such as axial elongation of the myopic eye, posterior staphyloma, chorio-retinal atrophy, and vitreous modifications such as PVD causing anteroposterior or tangential vitreous traction have been supposed to be causative factors [19]. Interestingly, changes in the macular thickness and retinal circulation inducing transient macular edema have been described to occur during the suction phase of the procedure. The macular hole formation seen in our series with intact posterior hyaloid can be explained by a combination of transient macular edema and transient anterior posterior vitreous traction [20-24].
A mechanism like the one proposed in cases of macular hole secondary to diabetic macular edema, where intraretinal fluid can promote dehiscence of the tissue, may be involved. Lin et al. reported a mean refractive error of -15.87 D in high myopic eyes that later developed MH [25]. Given that the eyes in our case series and almost all eyes in the literature review were myopic, it is hard to know if the patients would have developed MH regardless. However, the eyes in our case series had mild myopia, and lacked other risk factors for MH formation, including no trauma, PVD and intact hyaloid. Furthermore, the mean refractive error of the eyes in our literature review was considerably less than that reported by Lin. Based on this, we hypothesize that LASIK procedure may at least contribute further risk for MH development in myopic eyes. Following vitrectomy to correct the MH, visual outcomes appear to be good for most patients.
To our knowledge, this is the first review of the literature to describe the presentation and outcomes of MH formation following LASIK procedure. Additionally, our case series adds to the reports that LASIK procedure may be a potential risk factor for MH formation and that patients should be warned of this potential complication. Also, all reported cases demonstrated hole closure with vitrectomy and membrane peel surgery. The limitation of our study is small sample size (n of 2) making it difficult to draw conclusions on the causes of MH. The topic of LASIK and MH formation is not well researched with most reports coming from individual cases. Our case series and review demonstrate a potential association between LASIK and MH, and we recommend that future research focus on prospective studies to establish a definitive link between the two.
Conclusion
This case series and literature review evaluated the presentation and visual outcomes of patients who developed MHs following LASIK procedure. Our study showed that almost all patients were female and myopic, and developed unilateral MHs. Most patients lacked other risk factors for MH formation other than some myopia and had good visual outcomes following PPV surgery with or without ILM peel (but all had closure of MH with good anatomical outcome). In our case series, our patients improved following ILM peel with temporally hinged inverted ILM flap that was draped over the MH and was gently tucked into the MH. This surgical technique may offer an approach for cases where surgeons feel that simple ILM peel is insufficient for larger MHs; such that ILM tucking may offer an additional plugging and scaffolding effect for gliosis and better hole closure.
Myopia is known to be a risk factor for MH formation. However, LASIK procedure may potentially introduce further vitreofoveal traction.
Author Statements
Conflicts of Interest
None of the authors have any financial disclosures.
Authorship
All authors attest that they meet current ICMJE criteria for Authorship.
References
- Bikbova G, Oshitari T, Baba T, Yamamoto S, Mori K. Pathogenesis and management of macular hole: review of current advances. J Ophthalmol. 2019; 2019: 3467381.
- Ali FS, Stein JD, Blachley TS, Ackley S, Stewart JM. Incidence of and risk factors for developing idiopathic macular hole among a diverse group of patients throughout the United States. JAMA Ophthalmol. 2017; 135: 299-305.
- De Giacinto C, Pastore MR, Cirigliano G, Tognetto D. Macular hole in myopic eyes: A narrative review of the current surgical techniques. J Ophthalmol. 2019; 2019: 3230695.
- Kahuam-López N, Navas A, Castillo-Salgado C, Graue-Hernandez EO, Jimenez-Corona A, Ibarra A. Laser-assisted in-situ keratomileusis (LASIK) with a mechanical microkeratome compared to LASIK with a femtosecond laser for LASIK in adults with myopia or myopic astigmatism. Cochrane Database Syst Rev. 2020; 4: CD012946.
- Harasawa M, Quiroz-Mercado H, Salcedo-Villanueva G, Garcia-Aguirre G, Schwartz S. Inner segment ellipsoid band and cone outer segment tips changes preceding macular hole development in a young patient. Case Rep Ophthalmol Med. 2014; 2014: 132565.
- Bikbova G, Oshitari T, Sakurai M, Baba T, Yamamoto S. Macular hole after laser in situ keratomileusis in a 26-year-old patient. Case Rep Ophthalmol Med. 2013; 2013: 739474.
- García-Fernández M, Castro-Navarro J, Bajo-Fuente A. Vitreoretinal surgery for bilateral macular holes after laser-assisted in situ keratomileusis for the correction of myopia: a case report. J Med Case Rep. 2012; 6: 381.
- Arevalo JF, Mendoza AJ, Velez-Vazquez W, Rodriguez FJ, Rodriguez A, Rosales-Meneses JL, et al. Full-thickness macular hole after LASIK for the correction of myopia. Ophthalmology. 2005; 112: 1207-12.
- Ruiz-Moreno JM, Artola A, Pérez-Santonja JJ, Alió JL. Macular hole in a myopic eye after laser in situ keratomileusis. J Refract Surg. 2002; 18: 746-9.
- Chan CK, Lawrence FC. Macular hole after laser in situ keratomileusis and photorefractive keratectomy. Am J Ophthalmol. 2001; 131: 666-7.
- Lee SS, Lingham G, Sanfilippo PG, Hammond CJ, Saw SM, Guggenheim JA, et al. Incidence and progression of myopia in early adulthood. JAMA Ophthalmol. 2022; 140: 162-9.
- Rizzo S, Tartaro R, Barca F, Caporossi T, Bacherini D, Giansanti F. Internal limiting membrane peeling versus inverted flap technique for treatment of full-thickness macular holes: a comparative study in a large series of patients. Retina. 2018; 38: S73-8-s8.
- Baumann C, Kaye S, Iannetta D, Sultan Z, Dwivedi R, Pearce I. Effect of inverted internal limiting membrane flap on closure rate, postoperative visual acuity, and restoration of outer retinal layers in primary idiopathic macular hole surgery. Retina. 2020; 40: 1955-63.
- Yan Y, Zhao T, Sun C, Zhao H, Jia X, Wang Z. Anatomical and functional outcomes in eyes with idiopathic macular holes that underwent surgery using the inverted internal limiting membrane (ILM) flap technique versus the conventional ILM peeling technique. Adv Ther. 2021; 38: 1931-45.
- Ruiz-Moreno JM, Alió JL. Incidence of retinal disease following refractive surgery in 9,239 eyes. J Refract Surg. 2003; 19: 534-47.
- Arevalo JF, Ramirez E, Suarez E, Cortez R, Antzoulatos G, Morales-Stopello J, et al. Rhegmatogenous retinal detachment in myopic eyes after laser in situ keratomileusis. Frequency, characteristics, and mechanism. J Cataract Refract Surg. 2001; 27: 674-80.
- Mirshahi A, Schöpfer D, Gerhardt D, Terzi E, Kasper T, Kohnen T. Incidence of posterior vitreous detachment after laser in situ keratomileusis. Graefes Arch Clin Exp Ophthalmol. 2006; 244: 149-53.
- Krueger RR, Seiler T, Gruchman T, Mrochen M, Berlin MS. Stress wave amplitudes during laser surgery of the cornea. Ophthalmology. 2001; 108: 1070-4.
- Coppé AM, Ripandelli G, Parisi V, Varano M, Stirpe M. Prevalence of asymptomatic macular holes in highly myopic eyes. Ophthalmology. 2005; 112: 2103-9.
- Zhang J, Zhou YH. Effect of suction on macular thickness and retinal nerve fiber layer thickness during LASIK used femtosecond laser and Moria M2 microkeratome. Int J Ophthalmol. 2015; 8: 777-83.
- Chen M, Dai J, Gong L. Changes in retinal vasculature and thickness after small incision lenticule extraction with optical coherence tomography angiography. J Ophthalmol. 2019; 2019: 3693140.
- Zhang Y, Lan J, Cao D, Yang C, Yang D, Xie W, et al. Microvascular changes in macula and optic nerve head after femtosecond laser-assisted LASIK: an optical coherence tomography angiography study. BMC Ophthalmol. 2020; 20: 107.
- Ozsaygili C, Altunel O, Duru N. Evaluation of the change in retinal thickness after femtosecond laser-assisted laser in situ keratomileusis and photorefractive keratectomy. Curr Eye Res. 2022; 47: 18-24.
- Yoshida Y, Sato T, Oosuka S, Mimura M, Fukumoto M, Kobayashi T, et al. Two cases of diabetic macular edema complicated by an atypical macular hole. BMC Ophthalmol. 2020; 20: 171.
- Lin CW, Ho TC, Yang CM. The development and evolution of full thickness macular hole in highly myopic eyes. Eye (Lond). 2015; 29: 388-96.
- O’Leary éD, McNeillis B, Aybek S, Riordan-Eva P, David AS. Medically unexplained visual loss in a specialist clinic: a retrospective case-control comparison. J Neurol Sci. 2016; 361: 272-6.