
Case Report
Austin J Orthopade & Rheumatol. 2015;2(1): 1012.
Idiopathic Spinal Cord Herniation: Report of Two Cases
Amin M*, Zertalis M, Naidu L and Botchu R
Department of Radiology, Kettering General Hospital, UK
*Corresponding author: Amin M, Department of Radiology, Kettering General Hospital, Kettering, Rothwell road, Northamptonshire, NN16 8UZ, UK,
Received: November 20, 2014; Accepted: March 03, 2015; Published: March 05, 2015
Abstract
Idiopathic Spinal Cord Herniation (ISCH) is a rare condition that primarily affects the thoracic spine. It is increasingly recognised as a reversible cause of myelopathy. The condition predominantly affects middle aged adults and is characterised by an anterior dural tear through which the cord herniates. Despite its characteristic radiological appearances the condition is often misdiagnosed. We present two cases of ISCH and describe the treatment options. The literature review also discusses important diagnostic features.
Keywords: Idiopathic; Herniation
Abbreviations
ISCH: Idiopathic Spinal Cord Herniation; MRI: Magnetic Resonance Imaging
Introduction
The most common causes of spinal cord compression include injury, degenerative processes and masses. Idiopathic Spinal Cord Compression (ISCH) is an under-diagnosed cause of myelopathy. ISCH is a rare condition of progressive myelopathy originally described by Wortzman et al. [1]. The condition is characterised by a ventral dural defect through which the spinal cord herniates, most commonly in the thoracic region [2]. The pathogenesis of the condition remains unknown. ISCH is a potentially debilitating condition, which when managed appropriately can be cured. We review two cases of ISCH and discuss the common clinical presentations, diagnostic features, treatment options and patient outcomes.
Case 1
A 48-year-old female presented with right arm and left leg parathaesia. Her medical history included depression. There was no trauma prior to the onset of her symptoms, and the patient denied any sphincter disturbance. A cerebrovascular event was ruled out and she was discharged with neurology and neurosurgery outpatient followup. Over the next 18 months her symptoms alternated between the combination of right arm and left leg parathaesia to left arm and right leg parathaesia. During this time repeated examinations were generally normal, with an impaired sensation over C6-C8 dermatome being the only sign that was recorded once. Neurophysiological assessment was satisfactory and nerve conduction tests failed to identify a peripheral nerve lesion. The first MRI scan was reported as showing an anterior displacement of the cord at T4 related to an arachnoid cyst. Further imaging revealed that the abnormality was in fact an ISCH (Figure 1.1). The patient was also investigated for multiple sclerosis due to a visual disturbance and positive family history. The patient was managed conservatively, with follow up and remained neurologically stable.
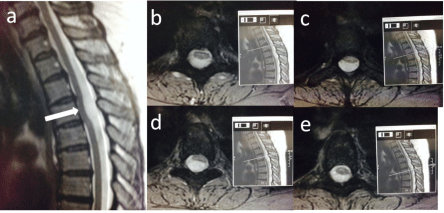
Figure 1.1: T2 sagittal (a) and axial (b-d) with corresponding sagittals
demonstrating anterior bowing of the thoracic cord.
Case 2
A 47-year-old male presented with pain in the right shoulder. His medical history included frequent urinary tract infections and nephrectomy. The pain was associated with clicking and the presence of electric shock like sensations. On examination there was no weakness or sensory deficits, and coordination and reflexes were intact. An initial MRI scan showed intradural high T2 abnormality at the level of T2 causing extrinsic compression on the spinal cord anteriorly. The patient was recalled for post gadolinium images which demonstrated a lack of enhancement at the level of T2 meaning that the abnormality was due to an anterior spinal cord herniation (Figure 1.2). The symptoms resolved spontaneously within a couple of months.
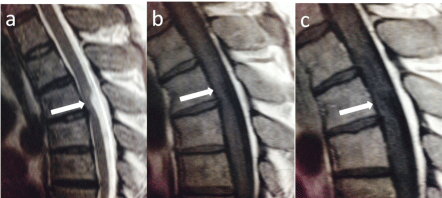
Figure 1.2: T2 sagittal (a); T1 (b) pre and T1 (c) post contrast sagittals
showing anterior bowing of the thoracic cord without abnormal enhancement.
Discussion
Wortzman et al [1] reported the first case of ISCH in 1974. The condition is characterised by a ventral dural defect of unknown pathogenesis, through which the spinal cord herniates and it is most frequently encountered between levels T3 and T7 [2]. Technological advances in Magnetic Resonance Imaging (MRI) have resulted in increased diagnoses. There are 175 cases of ISCH published to date [3]. It is approximately twice as common in females with a mean age of 49 years (range 12-75) at presentation. Interestingly, out of the 175 cases published to date there was only one paediatric case of ISCH reported by Goetti in a 12 year old girl [4].
ISCH is most frequently encountered clinically as Brown-Sequard syndrome, although other presentations such as monoparesis, paraparesis, sensory impairment, bowel and bladder dysfunction, impotence, chest and back pain have been reported [5-10]. Due to the rarity of the condition, in combination with the variability of clinical symptoms and misdiagnoses at presentation the mean duration of symptoms before a diagnosis of ISCH is established is 40 months [3,5]. Differential diagnoses at presentation include disc herniation, dorsal arachnoid cyst, transverse myelitis, arachnoiditis and intraor extra-dural mass [2,3,5]. A recent publication by Hawasli et al. [11] presented 5 more cases of ISCH that were misdiagnosed. As with the majority of cases in literature, these patients presented with varying asymmetric neurological signs. All had lower extremity weakness, and associated symptoms included pain, paraesthesia and incontinence. In one of the patients there was ISCH 5 years prior to the onset of symptoms before the progression of the abnormality led to neurological deficits. Case 2 in our report demonstrated no neurological signs that correlate with the patient’s MRI imaging. However, as with the patient reported by Hawasli et al. [11], the ISCH of case 2 may progress and lead to symptoms, equally there are patients that have remained non-progressive [12]. Case 1 had sensory impairments in her right leg which is often one of the first signs of ISCH [13]. There were no MRI findings to explain the neurological symptoms and signs of the upper limbs in both cases. Both cases in this report do not show advanced ISCH signs and according to the limited literature it is difficult to predict whether their ISCH will progress.
MRI is the investigation of choice for diagnosing ISCH with typical features demonstrated on both axial and sagittal images. Sagittal images demonstrate expansion of the dorsal subarachnoid space, and a ventrally displaced spinal cord with anterior C- or S-shaped angulation. On axial images there is antero-lateral displacement of the cord with loss of the normal intervening CSF signal [2-6]. These features were classically seen in our cases series. The commonest misdiagnosis reported in up to 45% of cases is a dorsal arachnoid cyst [14,15]. Phase contrast MRI demonstrating normal CSF flow dorsal to the herniated cord helps to exclude an obstructive lesion and confirm the diagnosis of ISCH.
ISCH can be managed surgically or conservatively. Surgical management involves reduction of the herniated spinal cord and closure of the ventral dural defect with sutures. Other surgical treatments described in literature include duraplasty, and widening of the dural defect [10,16,17]. Out of the 175 cases of ISCH, 160 patients received surgical treatment, and 15 patients were managed conservatively [2]. The main indications for surgical treatment were progressive myelopathy or the presence of a motor deficit. Bearing in mind the variable length of follow-up (1 – 216 months) and the absence of a validated outcome score, review of the literature identified that neurological outcome is reported as “improved”, “unchanged” or “worse”. The neurological outcome was unchanged for all patients that were managed conservatively. In the 160 patients that were managed surgically the neurological outcome improved for 120 (75%) patients, it remain unchanged for 28 (17.5%) patients, and 12 (7.5%) patients deteriorated following surgical treatment [2].
Conclusion – Teaching Point
ISCH is a rare cause of progressive myelopathy of unknown pathogenesis with variable clinical presentations but with typical features demonstrated on MRI. Sagittal images demonstrate expansion of the dorsal subarachnoid space, and an anteriorly angulated, ventrally displaced spinal cord. On axial images there is antero-lateral displacement of the cord with loss of the normal intervening CSF signal. Phase contrast MRI is crucial for excluding an obstructive lesion and confirming the diagnosis of ISCH.
References
- Wortzman G, Tasker RR, Rewcastle NB, Richardson JC, Pearson FG. Spontaneous incarcerated herniation of the spinal cord into a vertebral body: a unique cause of paraplegia. Case report. J Neurosurg. 1974; 41: 631-635.
- Groen RJM, Middel B, Meilof JF, Enting RH, Coppes MH, Journee LH, et al. Operative treatment of anterior thoracic spinal cord herniation: three new cases and an individual patient data meta-analysis of 126 case. 2009; 64: 145-159.
- Summers JC, Balasubramani YV, Chan PCH, Rosenfeld JV. Idiopathic spinal cord herniation: Clinical review and report of three cases. Asian J Neurosurg. 2013; 8: 97–105.
- Goetti R, Wille D, Kretzschmar U, Klein A, Scheer I. Idiopathic Spinal Cord Herniation. First Reported Case in a Child. JAMA Neurol. 2013; 70: 125-126.
- Sasani M, Ozer AF, Vural M, Sarioglu AL. Idiopathic Spinal Cord Herniation: Case Report and Review of the Literature. J Spinal Cord Med. 2009; 32: 86–94.
- Darbar A, Krishnamurthy S, Holsapple JW, Hodge CJ. Ventral thoracic spinal cord herniation: frequently misdiagnosed entity. Spine. 2006; 31: E600–E605.
- Miyaguchi M, Nakamura H, Shakudo M, Inoue Y, Yamano Y. Idiopathic spinal cord herniation associated with intervertebral disc extrusion: a case report and review of the literature. Spine. 2001; 26: 1090–1094.
- Senturk S, Guzel A, Guzel E. Atypical clinical presentation of idiophatic thoracic spinal cord herniation. Spine. 2008; 33: E474–E477.
- Vallée B, Mercier P, Menei P, Bouhour F, Fischer C, Fournier D, et al. Ventral transdural herniation of the thoracic spinal cord: surgical treatment in four cases and review of literature. Acta Neurochir (Wien). 1999; 141: 907–913.
- Ewald C, Kühne D, Hassler WE. Progressive spontaneous herniation of the thoracic spinal cord: case report. Neurosurgery. 2000; 46: 493–495.
- Hawasli A, Ray W, Wright N. Symptomatic thoracic spinal cord herniation: case series and technical report. Neurosurgery. 2014; 10: E498-E504.
- Massicotte EM, Montanera W, Ross Fleming JF, Tucker WS, Willinsky R, TerBrugge K, et al. Idiopathic spinal cord herniation: report of eight cases and review of the literature. Spine. 2002; 27: E233–E241.
- Berg-Johnson J, Ilstad E, Kolstad F, Mark Züchner, Jarle Sundseth. Idiopathic spinal cord herniation: an increasingly recognized cause of thoracic myelopathy. Central Nervous System Disease. 2014; 6: 85-91.
- Asgari S, Brondics A, Sandalcioglu IE, Schaefer H, Stolke D. Idiopathic herniation of the spinal cord: Review of the literature and own experiences. Akt Neurol. 2002; 29: 383–388.
- Blasel S, Hattingen E, Baas H, Zanella F, Weidauer S. Spontaneous spinal cord herniation: MR imaging and clinical features in six cases. Clin Neuroradiol. 2008; 18: 224–230.
- Chaichana KL, Sciubba DM, Li KW, Gokaslan ZL. Surgical management of thoracic spinal cord herniation: technical consideration. J Spinal Disord Tech. 2009; 22: 67–72.
- Wada E, Yonenobu K, Kang J. Idiopathic spinal cord herniation: report of three cases and review of the literature. Spine. 2000; 25: 1984–1988.