
Review Article
Austin J Orthopade & Rheumatol. 2015;2(2): 1017.
Musculoskeletal Ultrasound in Common Foot and Ankle Pathologies
Badon M1, Brown C2, Talusan PG3, Reach JS2 and Kohler MJ4*
1Department of Orthopedic Surgery, University of Massachusetts Memorial Medical Center, USA
2Department of Orthopedic Surgery, Yale University School of Medicine, USA
3Department of Orthopedic Surgery, University of Michigan, USA
4Department of Medicine, Division of Rheumatology,Allergy, Immunology, Harvard Medical School, USA
*Corresponding author: Minna J Kohler, Department of Medicine: Division of Rheumatology, Allergy,Immunology, Massachusetts General Hospital/Harvard Medical School, 55 Fruit St. Yawkey 2C, Boston MA 02114, USA
Received: April 06, 2015; Accepted: July 09, 2015; Published: July 11, 2015
Abstract
Background: Musculoskeletal ultrasound has been performed since the early 1970s, but a perceived high level of “operator dependence” limited its expansion into orthopaedic surgery. Recent advancements in hardware and image processing have given rise to lightweight portable machines capable of detailed examination of superficial bony and soft-tissue structures. This portability, coupled with other ultrasound advantages such as allowing dynamic, real-time, and functional evaluations without radiation exposure has stimulated interest in the use of ultrasound by orthopaedic surgeons.
Methods: This review examines the use of musculoskeletal ultrasound in the foot and ankle by the orthopaedic surgeon in the areas of tendon pathology, heel pain, inflammatory conditions, nerve pathology, fractures, sprains, and ultrasound-guided interventions.
Results: Musculoskeletal ultrasound has numerous advantages over standard imaging techniques in the diagnosis and treatment of musculoskeletal pathology. Musculoskeletal ultrasound fails, however, to adequately image deep pathologies in obese patients and joints hidden by bones.
Conclusion: Musculoskeletal ultrasound has many applications and it is necessary to continue the research of this imaging technology in other orthopaedic fields.
Keywords: Musculoskeletal ultrasound; Tendinopathy; Sprain; Plantar Fasciitis; Foot
Introduction
Musculoskeletal ultrasound (MUS) is an imaging technology that uses high-frequency sound waves to reconstruct an image. The transducer and image processor analyze sound waves reflected off of tissue interfaces. This produces a bright echo that determines relative location of structures as well as tissue size, shape, and consistency [1].
In the past decade, major advancements in the production and analysis of sonographic signals have resulted in improved resolution, less artifact, three and four-dimensional imaging, and extended fieldof- view reconstructions. Color and power Doppler ultrasound (US) are useful when examining synovium, vascular structures, effusions, and tumors [2].
A disadvantage of MUS is that it is operator dependent [3]. Like any other skill in orthopaedic surgery, with appropriate training and an orthopaedic surgeon’s deep understanding of musculoskeletal anatomy, physiology, and function, the learning curve may be lessened in this subspecialty group [4].
The ability to perform dynamic ultrasound imaging in the clinic, at the bedside, and in the operative theater (Figure 1) allows novel opportunities for the orthopaedic surgeon to enhance patient care in a cost-effective way [4]. MUS use has expanded rapidly among other musculoskeletal providers such as sports medicine, rheumatology, and physical medicine and rehabilitation. It is hoped that this review stimulates interest and research by orthopaedic surgeons in the use of this complementary imaging modality in the other orthopaedic subspecialties.
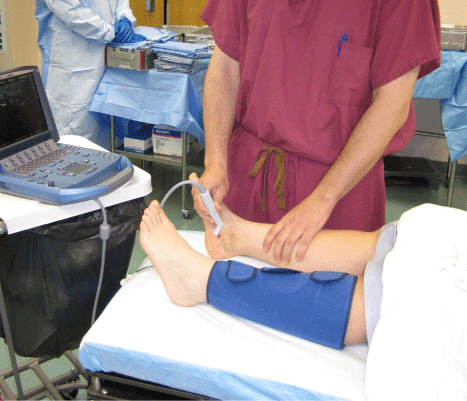
Figure 1: A hand-held musculoskeletal ultrasound in use. Note the small
size of the machine. Various transducers are available to provide discipline
specific imaging. Many ultrasound systems are as portable as a laptop and
may be taken by the surgeon from floor to clinic to operating room.
Tendon pathology
Tendons are organized bundles of longitudinal collagen fibers that appear hyperechoic or hypoechoic depending on the directionality of the ultrasound beam, a property called anisotropy. Given their superficial location, tendons of the lower extremity are readily evaluated by US [5] (Figure 2).
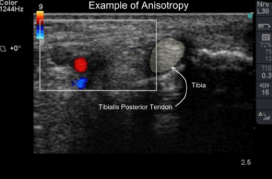
Figure 2: Short axis image though medial ankle structures. Tendons become
darker and brighter depending on their angulation with respect to transducer
orientation, a property termed anisotropy.
Changes in tendon caliber have been implicated as a marker of tendinopathy [6]. A prospective randomized study proposed a simple classification of tendinopathy to be a tendon twice the size on short axis imaging (where the transducer is perpendicular to the collagen fibers) as the contralateral normal tendon [4]. Using this classification, the authors defined which patients benefited from non-operative treatment (less than 2X normal size) and those who underwent surgical debridement and reconstruction (equal or greater than 2x normal size).
Achilles tendon
Nehrer performed ultrasounds on patients with pain in the Achilles region. None of the sonographically normal Achilles tendons ruptured whereas 28% of patients with sonographically abnormal, thickened tendons sustained a spontaneous Achilles rupture at an average of 48 months followup [7].
Achilles tendon rupture and insertional and nodular tendinopathies in athletes were described by Paavola in a study correlating preoperative US images with intraoperative findings [8]. US have found to be either equivalent or superior to MRI in diagnosing these Achilles pathologies. Pathognomonic US findings in insertional Achilles tendinopathy included prominent calcaneal exostosis (Haglund’s deformity), dynamic bone-tendon impingement, loss of organized collagen superstructure, and increased tendon diameter (Figure 3). Acute Achilles ruptures were well visualized by hypoechoic hematoma formation, gross tendon fiber disruption, and fat herniation through the deep crural fascia. Nodular tendinopathy was described as fusiform tendon enlargement on long axis imaging (where the transducer is parallel to the collagen fibers) 4 to 5 centimeters from the insertion point. Hypervascularity and florid arterial in-growth may be seen on color and power Doppler imaging.
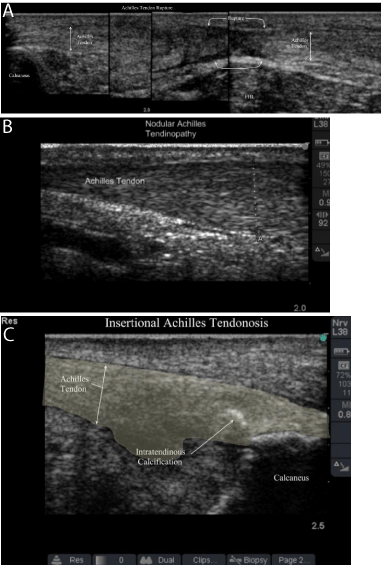
Figure 3: Achilles tendon pathology as seen on ultrasound. 3A: Achilles
tendon rupture; 3B: Nodular Achilles Tendinopathy with vascular in growth;
3C: Insertional Achilles Tendinopathy with hyperechoic calcifications and
hypoechoic lakes of tendinopathy.
Tibialis posterior tendon
Sonographic evaluation of the tibialis posterior tendon dysfunction (PTTD) shows thickening and heterogeneous hypoechoic echotexture with loss of normal fibrillar pattern in stage I PTTD [9]. Color and power Doppler US reveals hypervascularity and helps diagnose acute and chronic inflammation as well as thickening and involvement of the peritendinous soft tissues seen in peritendinitis [10].
Complete rupture of the tibialis posterior tendon is easily seen on US as an empty tibial groove when it occurs at the level of the medial malleous. Ruptured wavy fibril pattern tendon ends can be seen proximally and distally to the hypoechoic area of rupture (Figure 4). Harish showed that US can reliably define injury to the spring ligament and recommended US as first-line imaging in a patient with PTTD symptoms but normal tendon exam [9]. Kong et al. suggested that US imaging may help guide operative management of stage two and three PTTD by allowing dynamic testing of the tibialis posterior tendon, FDL tendon, and spring ligament complex [11].
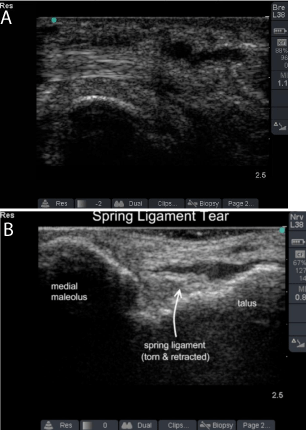
Figure 4: Posterior tibial tendinopathy and Spring Ligament tear.
(SL: Spring Ligament; T: Talus; PT: Posterior Tibialis Tendon)
Rockett compared US to MRI and found that US was more sensitivity and specific in diagnosing tibialis posterior and peroneal tendon tears [12].
In a blinded study, Gerling created longitudinal tears in cadaveric tibialis posterior tendons to examine the diagnostic efficiency of MRI and US in diagnosing tibialis posterior tendinopathy [13]. MR imaging had a sensitivity of 73%, specificity of 69%, and accuracy of 72%. Dynamic US had a sensitivity of 69%, specificity of 81%, and accuracy of 72%. Adding a static US examination increased the US specificity to 94%.
Peroneal tendons
Peroneal tendon tears and subluxation are extremely well visualized by MUS. In a prospective clinical study, Grant et al studied the sensitivity (100%), specificity (85%) and accuracy (90%) of US in correctly diagnosing surgically verified peroneal tendon pathology [14]. This study confirms our experience [4], that US should be considered the imaging modality of choice in the diagnosis of peroneal tendon pathology (Figure 5).
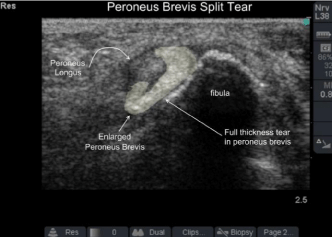
Figure 5: Peroneus brevis tendon split tear. On dynamic imaging the
peroneus longus tendon sandwiches the brevis between itself and the fibula:
sawing back and forth cutting the brevis in-line with its collagen bundles much
like a giggli saw.
Plantar fasciitis
Plantar fasciitis is the most common cause of plantar heel pain. Although the clinical diagnosis of plantar fasciitis is well described, the differential diagnosis for plantar pain is extensive [15]. Studies have focused on the ability of US to accurately diagnose plantar fasciitis and avoid costly confirmatory workups.
Static ultrasound imaging of plantar fasciitis shows an increased cross-sectional size of the plantar fascia. It also displays morphologic collagen fiber structural changes with lakes of hypoechoic areas disrupting the normal hyperechoic fibular structure of the plantar fascia [16]. Medial plantar facial thickness greater than 4mm in the setting of pathognomonic symptomatology is considered the general consensus for diagnosis of plantar fasciitis. An absolute thickness value cutoff, however, has proven contentious [17].
Sprains
Ankle sprains and instability are the most common athletic injury and may present as a spectrum of ligament attenuation, tendon tears, and avulsion fractures [18]. There are estimates that as much as 40% of ankle sprains may progress to chronic injury [19,20]. We commonly perform an “ultrasound palpation test” in cases of ankle and foot pain with normal radiographs. In this test, the maximal point of pain is identified and the underlying anatomic structures are viewed under US while palpating, manipulating, and stressing (Figure 6). In such cases, it is not uncommon to diagnose occult fracture of the lateral process of the talus, missed avulsion fractures, subluxating or split peroneal tendons, anterior talar osteochondral lesions, and anterolateral ankle impingement.
Figure 6: Fracture of the lateral process of the talus in mountain biker with
negative x-rays. Note the cortical discontinuity.
Ligamentous injuries are well imaged by US. In an outcomes based study, US accurately diagnosed deltoid ligament ruptures and changed management for patients in the setting of “fibularonly” ankle fractures [21]. This study suggested that US may offer a more attractive option than stress views or other advanced imaging modalities. Oae et.al followed up this result, directly comparing the accuracy of stress radiography, MUS, and MRI in the detection of ATFL injury in patients with confirmation by arthroscopy [22]. US have reported to be 91% accurate, compared to 67% accuracy for stress radiographs, and 97% accuracy for MRI.
Mei-Dan described and tested a standardized US examination for syndesmosis injury using dynamic US examination of AITFL by internal, external, and dorsiflexion manipulation of the foot and ankle [23]. Statistically significant differences were found between the injured and control group in the neutral, external rotation, and internal rotation positions (p < 0.001) and for the measured change between the AITFL in the external rotation and neutral positions (p < 0.01). One hundred percent sensitivity and specificity of US were reported in the diagnosis of syndesmosis injury using this technique.
Nerve Pathology and Space Occupying Lesions
Nerve entrapment syndromes present a challenging diagnosis when occurring in the foot and ankle [24, 25]. MUS helps define the anatomy and location of nerve entrapment, while simultaneously providing an opportunity to target image-guided procedures in the clinic or operative setting.
We have found US imaging useful in the diagnosis of compressive mass lesions. Ganglion cysts, for example, are particularly well visualized (Figure 6). In practice, we use a variant on the “ultrasound palpation test” (an “ultrasound Tinel’s” test) to help localize focal nerve compression, injuries, and neuromas. When used perioperatively and intraoperatively, such ultrasound guidance appears to help limit operative dissection and limit collateral comorbidity.
The use of US has been examined in the evaluation of tarsal tunnel syndrome [26]. Intraoperative findings were found to be consistent with preoperative ultrasound findings with no false-negative results. Furthermore, US accurately diagnosed the underlying cause of tarsal tunnel syndrome in all cases in this study. The authors suggested US to be a surgeon-friendly imaging modality, complementary to MRI in the evaluation and treatment of tarsal tunnel syndrome.
An “ultrasound Mulder’s sign” [27] or “click” has been proposed as a way to confirm the diagnosis of a Morton’s neuroma. Perini et.al compared the diagnostic effectiveness of physical examination, static US, dynamic US examination using the “ultrasound Mulder’s click”, and MRI with findings at surgery used to judge diagnostic success [28]. A sensitivity of 65% for static US, 100% for dynamic US, and 72.7% for MRI for Morton’s neuroma was reported. US imaging provided evidence of an alternate diagnosis that changed operative management when a neuroma was not visualized. Based on these findings, the authors concluded that dynamic US should be used as the gold standard imaging modality for the evaluation of Morton’s neuromas.
Ultrasound-guided Interventions
MUS has shown excellent accuracy in guiding injection treatments of inflammatory conditions, joints, and tendon sheaths. Reach et.al performed a blinded cadaver study to assess the accuracy of US-guided injections in the foot and ankle. US-guided injections were found to be 100% accurate in six sites (first MTP joint, second MTP joint, tibiotalar joint, Achilles peritendinous space, flexor hallucis longus sheath, tibialis posterior tendon sheath); subtalar joint injections were 90% accurate [29].
US-guided FHL injection was successful in 24 of 24 injections in a study by Mehdizade and Adler. They advocated that direct visualization of the needle tip could help avoid injury to the adjacent neurovascular bundle [30].
Balint et al. compared blind versus US-guided aspirations in 64 patients with inflammatory arthropathy. Successful aspiration was defined by the ability to remove a recordable amount of synovial fluid [31]. Blind ankle aspirations were 20% successful versus 100% successful in the US-guided group. Interestingly, 0% of the blind small-joint aspirations were successful in this study, while US guidance yielded a 100% success rate.
US-guided injections have been found effective for Morton’s neuromas, resulting in excellent pain and satisfaction outcome scores in the relief of symptoms [32].
Inflammatory Condition
The use of US in the diagnosis of inflammatory heel pain has been studied, finding similar increased thickening in have plantar fasciae of idiopathic plantar fasciitis patients and rheumatoid patients with acute enthesopathy (p<0.001) [33]. Based on this study, US imaging was recommended to be used as an essential part of the workup of patients suffering inflammatory arthropathies and foot pain.
Newman et al confirmed a correlation between pain level (as determined by a visual analogue scale) and degree of hyperemia seen on power Doppler imaging in a controlled study of synovitis [34].
We have found power Doppler US useful in the initial diagnosis and in follow-up monitoring throughout treatment in patients with recalcitrant inflammatory conditions. US images of tenosynovitis characteristically reveal hypoechoic thickening of the tendon and tendon sheath. Color and Power Doppler can aid in differentiating synovial sheath thickening and synovitis from synovial sheath effusion. The rheumatology literature shows growing interest in the use of power Doppler US for a wide variety of inflammatory conditions including rheumatoid arthritis [35], enthesitis related to spondyloarthropathies [36,37] and gout [38].
Fractures
US have been shown to be useful in diagnosing fractures in the foot and ankle that may not be visible on standard radiographs [39]. Up to 85% of stress fractures may be overlooked on initial radiograph with up to 50% subsequently missed on second radiograph [40]. Woodward et al. proposed US guidelines for the diagnosis of occult Lisfranc injury [41]. A tear in the dorsal ligament-capsule between the medial and second metatarsal, along with hematoma and widening of the interosseus space greater than 2.5mm on dynamic weight-bearing US, confirmed the diagnosis of a Lisfranc disruption. We have found US useful in early detection of commonly missed foot and ankle fractures. On US, fractures appear as discontinuities in the hyperechoic surface of the cortex, avulsion fractures, hematoma, surrounding edema, and ligamentous disruptions are commonly seen (Figure 6).
Banal et al. performed a case-control study examining the sensitivity (83%) and specificity (76%) of US in the diagnosis of metatarsal stress fractures in the setting of negative x-rays. They proposed a new imaging algorithm in which US is used after plain film x-rays when metatarsal stress fracture is suspected [42]. Gregg et al. performed a prospective clinical and cadaver study examining the use of ultrasound to evaluate plantar plate pathology [43]. Plantar plate tears were visualized on US by the presence of hypoechoic defects punctuating the normally homogeneous hyperechoic background of the plantar plate. Color and power Doppler US was used to show increased vascularity in both acute and chronic plantar plate injury. Using MRI as a reference standard, 91% sensitivity and 44% specificity for plantar plate disruption were reported. We have found that the use of dynamic US and stress examination under US (an “ultrasound toe Lachman” test) aids in the evaluation of underlying structural pathology in patients suffering metatarsalgia.
In the pediatric population, US allow the visualization of cartilage growth plates while avoiding exposure to ionizing radiation. Simanovsky et al. performed US examinations on children with clinical and examination findings suspicious for foot and ankle fracture but negative plain film images [44]. Occult fractures were identified in 35% of these patients. The presence of periosteal reaction, cortical disruption, and hypoechoic surrounding tissues were found in suspected pediatric fracture cases. High diagnostic accuracy of US for femur and humerus fractures led to a proposed US-based algorithm for the evaluation of suspected fractures in children [45].
Conclusion
There is growing interest in musculoskeletal US as a diagnostic and therapeutic instrument across multiple specialties. Orthopaedic surgeons have an integral role to play in the advancement of this field. Despite this increasing interest in MUS, clear limitations exist to its application to all musculoskeletal pathology. Adipose tissue attenuates high frequency signal, making obese patients with deep structural pathology an imaging challenge. While superficial bony structure is seen in detail, current ultrasound machines fail to show what lies beneath the bones. The majority of joints are hidden by bony obstruction as well.
Nevertheless, MUS has considerable advantages for orthopaedic surgeons, as well as other musculoskeletal providers, and their patients. Dynamic US examinations of the musculoskeletal system afford optimal, on-the-fly modification of patient positioning and imaging technique to assess relevant anatomy and pathology. Images performed by the orthopaedic surgeon at the initial consultation (or operative intervention) provide immediate, real-time diagnostic results that are demonstrable to the patient, and assists with treatment planning and counseling. US has safe, cost-effective, and has no ionizing radiation exposure.
Criticisms regarding operator-dependence, measurement reliability, and image reproducibility will likely subside as standardization of evidence-based scanning protocols emerge and education/ training standards for US proficiency are further developed. Precedents for US imaging standards can be found in such disparate fields as obstetrics (the biophysical profile of a fetus), vascular surgery (carotid duplex examination for carotid stenosis), emergency medicine (FAST exam), and endocrinology (thyroid imaging and guided biopsy). Standard US imaging protocols for MUS are currently being taught through various radiology, physiatry, and rheumatology US courses. Efforts to implement US training programs in undergraduate and graduate medical education are steadily being promoted.
MUS have the potential to improve individual patient care and decrease healthcare costs. We have the obligation to ensure that research performed in the area meets the highest level of peer-review.
Recently, the American Institute of Ultrasound in Medicine (AIUM) invited representatives from over 50 medical and surgical societies to draft guidelines for the performance of MUS. Such guidelines may offer us the opportunity to improve clinical reproducibility and may prove a valuable tool to measure progress in future orthopaedic research.
References
- Whittaker JL, Teyhen DS, Elliott JM, Cook K, Langevin HM, Dahl HH, et al. Rehabilitative ultrasound imaging: understanding the technology and its applications. J Orthop Sports Phys Ther. 2007; 37: 434-449.
- Jacobson JA. Fundamentals of Musculoskeletal Ultrasound. Philadelphia PA: Saunders/Elsevier. 2007.
- Backhaus M, Burmester GR, Gerber T, Grassi W, Machold KP, Swen WA, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Ann Rheum Dis. 2001; 60: 641-649.
- Reach JS, Easley ME, Nunley JA. Classification of tendinopathy based on cross-sectional tendon size: ultrasound correlation with operative findings. Eastern & Southern Orthopaedic Association Proceedings. 2007; 24: 20-21.
- Reach JS, Nunley JA. Ultrasound Examination of the Achilles Tendon. In: The Achilles Tendon Treatment & Rehabilitation. Nunley J. NY, Springer Science. 2009.
- Campbell RS, Grainger AJ. Current concepts in imaging of tendinopathy. Clin Radiol. 2001; 56: 253-267.
- Nehrer S, Breitenseher M, Brodner W, Kainberger F, Fellinger EJ, Engel A, et al. Clinical and sonographic evaluation of the risk of rupture in the Achilles tendon. Arch Orthop Trauma Surg. 1997; 116: 14-18.
- Paavola M, Paakkala T, Kannus P, Järvinen M. Ultrasonography in the differential diagnosis of Achilles tendon injuries and related disorders. A comparison between pre-operative ultrasonography and surgical findings. Acta Radiol. 1998; 39: 612-619.
- Harish S, Jan E, Finlay K, Petrisor B, Popowich T, Friedman L, et al. Sonography of the superomedial part of the spring ligament complex of the foot: a study of cadavers and asymptomatic volunteers. Skeletal Radiol. 2007; 36: 221-228.
- Premkumar A, Perry MB, Dwyer AJ, Gerber LH, Johnson D, Venzon D, et al. Sonography and MR imaging of posterior tibial tendinopathy. AJR Am J Roentgenol. 2002; 178: 223-232.
- Kong A, Van Der Vliet A. Imaging of tibialis posterior dysfunction. Br J Radiol. 2008; 81: 826-836.
- Rockett MS, Waitches G, Sudakoff G, Brage M. Use of ultrasonography versus magnetic resonance imaging for tendon abnormalities around the ankle. Foot Ankle Int. 1998; 19: 604-612.
- Gerling MC, Pfirrmann CW, Farooki S, Kim C, Boyd GJ, Aronoff MD, et al. Posterior tibialis tendon tears: comparison of the diagnostic efficacy of magnetic resonance imaging and ultrasonography for the detection of surgically created longitudinal tears in cadavers. Invest Radiol. 2003; 38: 51- 56.
- Grant TH, Kelikian AS, Jereb SE, McCarthy RJ. Ultrasound diagnosis of peroneal tendon tears. A surgical correlation. J Bone Joint Surg Am. 2005; 87: 1788-1794.
- Neufeld SK, Cerrato R. Plantar fasciitis: evaluation and treatment. J Am Acad Orthop Surg. 2008; 16: 338-346.
- Cardinal E, Chhem RK, Beauregard CG, Aubin B, Pelletier M. Plantar fasciitis: sonographic evaluation. Radiology. 1996; 201: 257-259.
- McMillan AM, Landorf KB, Barrett JT, Menz HB, Bird AR. Diagnostic imaging for chronic plantar heel pain: a systematic review and meta-analysis. J Foot Ankle Res. 2009; 2: 32.
- Garrick JG. Characterization of the patient population in a sports medicine facility. Physician Sportsmed. 1985; 13: 73-76.
- Cass JR, Morrey BF, Katoh Y, Chao EY. Ankle instability: comparison of primary repair and delayed reconstruction after long-term follow-up study. Clin Orthop Relat Res. 1985; 110-117.
- Mizel MS, Hecht PJ, Marymont JV, Temple HT. Evaluation and treatment of chronic ankle pain. Instr Course Lect. 2004; 53: 311-321.
- Chen PY, Wang TG, Wang CL. Ultrasonographic examination of the deltoid ligament in bimalleolar equivalent fractures. Foot Ankle Int. 2008; 29: 883- 886.
- Oae K, Takao M, Uchio Y, Ochi M. Evaluation of anterior talofibular ligament injury with stress radiography, ultrasonography and MR imaging. Skeletal Radiol. 2010; 39: 41-47.
- Mei-Dan O, Kots E, Barchilon V, Massarwe S, Nyska M, Mann G. A dynamic ultrasound examination for the diagnosis of ankle syndesmotic injury in professional athletes: a preliminary study. Am J Sports Med. 2009; 37: 1009- 1016.
- Bora FW Jr, Osterman AL. Compression neuropathy. Clin Orthop Relat Res. 1982; 20-32.
- Schon LC. Nerve entrapment, neuropathy, and nerve dysfunction in athletes. Orthop Clin North Am. 1994; 25: 47-59.
- Nagaoka M, Matsuzaki H. Ultrasonography in tarsal tunnel syndrome. J Ultrasound Med. 2005; 24: 1035-1040.
- Torriani M, Kattapuram SV. Technical innovation. Dynamic sonography of the forefoot: The sonographic Mulder sign. AJR Am J Roentgenol. 2003; 180: 1121-1123.
- Perini L, Del Borrello M, Cipriano R, Cavallo A, Volpe A. Dynamic sonography of the forefoot in Morton’s syndrome: correlation with magnetic resonance and surgery. Radiol Med. 2006; 111: 897-905.
- Reach JS, Easley ME, Chuckpaiwong B, Nunley JA 2nd. Accuracy of ultrasound guided injections in the foot and ankle. Foot Ankle Int. 2009; 30: 239-242.
- Mehdizade A, Adler RS. Sonographically guided flexor hallucis longus tendon sheath injection. J Ultrasound Med. 2007; 26: 233-237.
- Balint PV, Kane D, Hunter J, McInnes IB, Field M, Sturrock RD. Ultrasound guided versus conventional joint and soft tissue fluid aspiration in rheumatology practice: a pilot study. J Rheumatol. 2002; 29: 2209-2213.
- Markovic M, Crichton K, Read JW, Lam P, Slater HK. Effectiveness of ultrasound-guided corticosteroid injection in the treatment of Morton’s neuroma. Foot Ankle Int. 2008; 29: 483-487.
- Gibbon WW, Long G. Ultrasound of the plantar aponeurosis (fascia). Skeletal Radiol. 1999; 28: 21-26.
- Newman JS, Laing TJ, McCarthy CJ, Adler RS. Power Doppler sonography of synovitis: assessment of therapeutic response--preliminary observations. Radiology. 1996; 198: 582-584.
- Wakefield RJ, Freeston JE, O’Connor P, Reay N, Budgen A, Hensor EM, et al. The optimal assessment of the rheumatoid arthritis hindfoot: a comparative study of clinical examination, ultrasound and high field MRI. Ann Rheum Dis. 2008; 67: 1678-1682.
- D’agostino MA, Aegerter P, Jousse-Joulin S, Chary-Valckenaere I, Lecoq B, Gaudin P, et al. How to evaluate and improve the reliability of power Doppler ultrasonography for assessing enthesitis in spondylarthritis. Arthritis Rheum. 2009; 61: 61-69.
- Balint PV, Sturrock RD. Inflamed retrocalcaneal bursa and Achilles tendonitis in psoriatic arthritis demonstrated by ultrasonography. Ann Rheum Dis. 2000; 59: 931-933.
- Thiele RG, Schlesinger N. Diagnosis of gout by ultrasound. Rheumatology (Oxford). 2007; 46: 1116-1121.
- Wang CL, Shieh JY, Wang TG, Hsieh FJ. Sonographic detection of occult fractures in the foot and ankle. J Clin Ultrasound. 1999; 27: 421-425.
- Stafford SA, Rosenthal DI, Gebhardt MC, Brady TJ, Scott JA. MRI in stress fracture. AJR Am J Roentgenol. 1986; 147: 553-556.
- Woodward S, Jacobson JA, Femino JE, Morag Y, Fessell DP, Dong Q. Sonographic evaluation of Lisfranc ligament injuries. J Ultrasound Med. 2009; 28: 351-357.
- Banal F, Gandjbakhch F, Foltz V, Goldcher A, Etchepare F, Rozenberg S, et al. Sensitivity and specificity of ultrasonography in early diagnosis of metatarsal bone stress fractures: a pilot study of 37 patients. J Rheumatol. 2009; 36: 1715-1719.
- Gregg J, Silberstein M, Schneider T, Marks P. Sonographic and MRI evaluation of the plantar plate: A prospective study. Eur Radiol. 2006; 16: 2661-2669.
- Simanovsky N, Lamdan R, Hiller N, Simanovsky N. Sonographic detection of radiographically occult fractures in pediatric ankle and wrist injuries. J Pediatr Orthop. 2009; 29: 142-145.
- Hübner U, Schlicht W, Outzen S, Barthel M, Halsband H. Ultrasound in the diagnosis of fractures in children. J Bone Joint Surg Br. 2000; 82: 1170-1173.