
Review Article
Austin J Orthopade & Rheumatol. 2016; 3(1): 1029.
Long Term Effects of Hyaluronate Viscosupplementation on Th17 and Activated CD4+ T Lymphocytes Blood Expression in Patients with Hip or Knee Osteoarthritis
Lurati A*, Laria A and Scarpellini M
Rheumatology Unit, Fornaroli Hospital Magenta, Italy
*Corresponding author: Lurati A, Rheumatology Unit, Fornaroli Hospital Magenta, Magenta, Italy
Received: April 14, 2016; Accepted: June 03, 2016; Published: June 08, 2016
Abstract
Background: Osteoarthritis (OA) is a chronic disease associated with joint destruction that has long been regarded in the past as a “no inflammatory” disease.
Aim of our study was to measure in the long term characteristics of T lymphocytes CD4+ Th1 Th2 and Th17 in peripheral blood of patients with hip or knee OA treated with viscosupplementation.
Methods: Patients with early primary knee or hip OA (ACR Criteria) who completed the 3 months study about the peripheral T lymphocytes concentration as described from our study group(Osteoarthritis Cartilage. 2015 Jan;23(1):88-93) entered this long-term extension. After 6 months from the last observation (i.e. 9 months check-point from baseline), a new flow-cytometry from blood samples to assess T cells subpopulations (CD3, CD4, CD8, CCR6, CD38, CxCR3, and HLA DR) was performed. Lequesne index was scored and compared to data at baseline and at the end of the original study.
Results: 35 patients treated were recruited (24 knee OA, 11hip OA). Data of 15 subjects with OA in the control untreated group were collected too. In the treated group, Th 17, activated CD4 T cells levels, Th2, Th1were similar to those observed after 3 months from viscosupplementation course (p=0.07), persistently higher than in control group (p=0.01). Lequesne index was also significantly higher between treated and control groups (p=0.01).
Conclusion: Our results seem to show that HA injections maintain low levels of activated T cells in patients with hip or knee OA. Alongside also the severity indices show a similar trend.
Introduction
Osteoarthritis (OA) is the most frequent rheumatic disease affecting up to 6% of the population, accounting for up to 20% of consultations at primary care level and is a leading cause of disability at work. OA is characterized by joint pain and dysfunction due to progressive subchondral bone damage, articular cartilage loss, inflammation/synovitis, and osteophyte formation [1,2].
Hyaluronic Acid (HA) is a long chain polysaccharide present in loose connective tissue, skin, the eye and synovial fluid where it secreted continuously by the synovial membrane into the joint space and comprises the major macro-molecular part of the synovial fluid. It is highly concentrated at the surface of the articular cartilage and the superficial layers of the synovial membrane. In the synovial fluid, HA acts as both a lubricant and a shock absorber during compressive stress [3,4].
Osteoarthritis is associated with a decrease in concentration and degradation with lowering in molecular weight of native HA in synovial fluid, ultimately leading to reduced viscoelastic properties of synovial fluid and induction of proinflammatory pathways [5].
Humoral and cellular immunity, both innate and adaptive immune response, are known to be involved. In particular, in many studies on OA, it has been demonstrated that an inflammatory synovium/synovitis has linked to increased cartilage damage and pain [6-8].
Intraarticular injection of exogenous HA replace the OA-induced deficit via multiple pathways (e.g. inhibition of degradative enzymes and inflammatory processes, stimulation of chondrocyte metabolism, and synthesis of articular cartilage matrix components) [9,10].
In previous studies our group demonstrated an elevation in activated T lymphocytes levels (as well as observed in rheumatoid arthritis, but to a lesser degree) as mark of a systemic pro inflammatory milieu in patients with OA. In these patients a single course of 3 intrarticular injection of HA showed effectiveness in lowering activation intensity [11].
Now, we show the long term data about lymphocytes levels and severity index (Lequesne) in patients who completed the 3 months observational study as described by our own group
Methods
Patients with early primary knee or hip OA (ACR Criteria) who completed the 3 months study about the peripheral T lymphocytes concentration (Osteoarthritis Cartilage. 2015 Jan;23 (1):88-93. Epub 2014 Sep 22) entered this long-term extension. After 6 months from the last observation (i.e. 9 months check-point from baseline), a new flow-cytometry from blood samples to assess T cells subpopulations (CD3, CD4, CD8, CCR6, CD38, CxCR3, HLA DR) was performed. Lequesne index was scored and compared to data at baseline and at the end of the original study. All patients, included controls, read, understood and signed an informed consent and compiled themselves a pain-functional Lequesne Index. The trial was conducted in accordance with the ethics principles of the Declaration of Helsinki and was approved by the local research ethics committees. No change in analgesics, non-steroidal anti-inflammatory drugs (NSAIDs), symptomatic slow acting drugs for OA (SYSADOAs), disease modifying (DMOADs) or intraarticular treatment was permitted throughout this extension period of the study.
Flow cytometry
Freshly drawn EDTA blood samples were analyzed by 8-color flow cytometry (FACSCanto II, Becton Dickinson, Milan) with the following conjugated antibody panel: CD45-FITC; CXCR3-PE; CD4-PerCP-Cy5.5; CCR6-PE-Cy7; CD38-Alexa 647; CD8-APC-H7; CD3-V450; HLADR-V500 at the appropriate concentrations (all from Becton Dickinson). After 20-minute staining in the dark, 2ml of ammonium chloride lysing was added for 10 minutes. After centrifugation at 1500 rpm for 7 minutes, the pellet was resuspended in 200 microliters of cold PBS and immediatalyanalyzed.
At least 50,000 lymphocytes (defined as CD45+++, SSClow cells) were acquired.
The gating strategy included the parallel capture of CD4+/CD3+ and CD8+/CD3+ cells in two separate downstream hierarchies. Each parent subset was then further dissected into functional subpopulations, namely CD4+ T cells as Th1 cells (CD4+ CXCR3+ CCR6-), Th2 cells (CD4+ CXCR3- CCR6-) and Th17 cells (CD4+ CXCR3- CCR6+), respectively according to Maecker et al. 20128. Both CD4+ and CD8+ cells were divided into quiescent (CD38- HLADR-) or activated elements (CD38+ and/or HLADR+). Functional subset percentages were calculated over the total lymphocyte population and over the parent CD4+ or CD8+ subsets, respectively, and all values were also recorded as absolute levels per microliter on the basis of total lymphocyte count (Beckman Coulter DXH800, Milan).
Statistical analysis
Normal distribution was stated by Shapiro-Wilk test. Homoscedasticity of variances was assessed with Cochran/Hartley or Levene tests. Continuous variables were presented as mean (±SD when suitable), and compared using two-tailed Student’s t-test or one-way Analysis Of Variance (ANOVA). Statistical analyses were performed with SPSS version 20 (SPSS Inc., Chicago, USA) for Windows. P-values<0.05 were considered statistically significant.
Results
35 patients were recruited (24 knee OA, 11hip OA). Data about 15 subjects in control group were collected too. Demographics in (Table 1). In the treated group, Th 17, activated CD4 T cells levels (CD4+CD38+DR+, CD4+CD38-DR+), Th2 (CD4+CXCR3-CCR6-), Th1 (CD4+CXCR3+CCR6-) were similar to those observed after 3 months from viscosupplementation course (p=0.07), persistently higher than in control group (p=0.01) (Figure 1, Table 2). In the treated group, Lequesne index was significantly higher at baseline than at 3 and 9 months check-point (p=0.01), no difference was present between these two, instead (p=0.1). Comparing data with control group, a significantly higher value was observed (p=0.03) (Figure 2).
KNEE OA (treated)
HIP OA (treated)
OA CONTROLS (untreated)
p
TOTAL PATIENTS
24 pts
11 pts
15 pts
AGE (±SD)
66.1± 1.1 years old
68.2 years old ±2.1 years old
67± 0.2 years old
0.2
SEX (Female)
21 pts
9 pts
12 pts
0.3
Median BMI (±SD)
23.41 (± 3.14)
24.30 (± 2.7)
26.4 (±3.44)
0.1
Table 1: Demographic data of whole population.
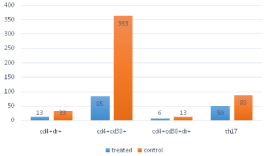
Figure 1: The cells levels in the 2 groups.
T cell type
Treated group
Control group
P value
CD4+
654±123
976 ±267
0.11
Th1
164±61
273±135
0.08
Th2
93±19
367±148
0.07
Th17
50±26
88±31
0.01
CD4+DR+
13±4
33±32
0.01
CD4+CD38+
85±32
363±40
0.03
CD4+CD38+DR+
6±3
13±10
0.02
Table 2: T cells levels in the 2 groups.
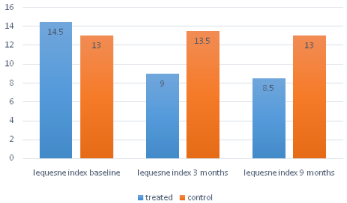
Figure 2: Lequesne index in the 2 groups.
Discussion
Numerous clinical trials have led to inclusion of HA in many major published guidelines for treatment of OA [12-14].
The mechanism of action of administered intra-articular HA is not completely understood, but as its clinical benefit exceeds its intraarticular presence, it is thought to induce production of HA and other extracellular matrix components and in particular suppress the inflammatory response and inhibit substance P. HA could bind to specific receptors expressed in many cells, such as the Cluster Determinant 44 (CD44), the Intracellular Adhesion Molecule-1 (ICAM-1) and the Receptor For Hyaluronate-Mediated Motility (RHAMM) [15,16].
This binding triggers various intracellular signal events such as cytokine release and stimulation of cell cycle proteins. The consequences of these interactions are to stimulate a pro inflammatory articular milieu [17-19].
Up to date in literature only the AMELIA study (a multicentre, randomised, patient and evaluator-blinded, controlled study in 306 patients with knee osteoarthritis) compared against placebo the efficacy and safety of repeated injections of Hyaluronic Acid (HA) over 40 months. In this study, at the 40-month visit significantly more patients responded to HA compared with placebo [20].
Our data suggest a prolonged effect of HA both clinical and laboratory at least until 6 months after an intraarticular course. Up to date our study with this time-extension is the first one specifically designed to measure the variations over time of OA T cells phenotype after HA injections and to relate it to a clinical outcome measure as the Lequesne index.
The present data confirm that th17 and activated T cells are persistently higher in blood samples from untreated patients with knee or hip OA compared with subjects treated with HA. According to data literature, our study found a significant clinical effect of HA in terms of Lequesne index.
These data suggest not only a local time-limited anti-inflammatory effect of HA but also a disease modifying action as a DMOADs in the joint milieu resulting in a decrease in pro inflammatory T cells concentrations, a restoration the rheological properties of synovial fluid and in a clinically less severe disease. Due to the low sample size of our study, further large scale prospective placebo-controlled studies, coupling biomarkers and imaging techniques are needed to confirm these results and to investigate furthermore the possible disease modifying effect of HA as suggested in this work.
References
- 1. Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005; 365: 965-973.
- 2. Felson DT. An update on the pathogenesis and epidemiology of osteoarthritis. Radiol Clin North Am. 2004; 42: 1-9.
- 3. Dahl LB, Dahl IM, Engström-Laurent A, Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985; 44: 817-822.
- 4. Balazs EA, Watson D, Duff IF, Roseman S. Hyaluronic acid in synovial fluid. I. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis Rheum. 1967; 10: 357-376.
- 5. Pelletier JP, Martel-Pelletier J. The pathophysiology of osteoarthritis and the implication of the use of hyaluronan and hylan as therapeutic agents in viscosupplementation. J Rheumatol Suppl. 1993; 39: 19-24.
- 6. Tsuruha J, Masuko-Hongo K, Kato T, Sakata M, Nakamura H, Sekine T, et al. Autoimmunity against YKL-39, a human cartilage derived protein, in patients with osteoarthritis. J Rheumatol. 2002; 29: 1459-1466.
- 7. Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritisd results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005; 13: 361-367.
- 8. Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007; 66: 1599-1603.
- 9. Smith MM, Ghosh P. The synthesis of hyaluronic acid by human synovial fibroblasts is influenced by the nature of the hyaluronate in the extracellular environment. Rheumatol Int. 1987; 7: 113-122.
- 10. Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005; 13: 216-224.
- 11. Lùrati A, Laria A, Mazzocchi D, Re KA, Marrazza M, Scarpellini M. Effects of Hyaluronic Acid (HA) viscosupplementation on peripheral Th cells in knee and hip osteoarthritis. Osteoarthritis Cartilage. 2015; 23: 88-93.
- 12. Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. A meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2004; 86: 538-545.
- 13. Peyron JG. A new approach to the treatment of osteoarthritis: viscosupplementation. Osteoarthritis Cartilage. 1993; 1: 85-87.
- 14. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006; CD005321.
- 15. Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, et al. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997; 159: 2492-2500.
- 16. Siegelman MH, DeGrendele HC, Estess P. Activation and interaction of CD44 and hyaluronan in immunological systems. J Leukoc Biol. 1999; 66: 315-321.
- 17. Cao JJ, Singleton PA, Majumdar S, Boudignon B, Burghardt A, Kurimoto P, et al. Hyaluronan increases RANKL expression in bone marrow stromal cells through CD44. J Bone Miner Res. 2005; 20: 30-40.
- 18. Iannitti T, Rottigni V, Palmieri B. A pilot study to compare two different hyaluronic acid compounds for treatment of knee osteoarthritis. Int J Immunopathol Pharmacol. 2012; 25: 1093-1098.
- 19. McGrath AF, McGrath AM, Jessop ZM, Gandham Surya, Datta G, S Dawson-Bowling, et al. Comparison of Intra-Articular Hyaluronic Acid Competitors in the Treatment of Mild to Moderate Knee Osteoarthritis J Arthritis. 2013; 2.
- 20. Navarro-Sarabia F, Coronel P, Collantes E, Navarro FJ, de la Serna AR, Naranjo A, et al. AMELIA study group. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: the AMELIA project. Ann Rheum Dis. 2011; 70: 1957-1962.