
Research Article
Austin J Orthopade & Rheumatol. 2018; 5(1): 1061.
Effectiveness and Safety of a Combination of Intra- Articular Corticosteroid and Local Anesthetic in Indian Patients with Knee Osteoarthritis: A Pilot Study
Jagdish RK¹*, Bhatnagar MK², Malhotra A³ and Shailly4
¹Medicine and Incharge Rheumatology Clinic, Santosh Medical College & Hospital, Ghaziabad and Max and Kailash Hospital, India
²Santosh Medical College & Hospital, Ghaziabad and Lady Harding Medical College, India
³Santosh Medical College and Hospital, Ghaziabad, India
4Chest Medicine, Chest and TB Hospital, Govt. Medical College, Patiala, India
*Corresponding author: Jagdish RK, Consultant Rheumatologist, Kailash and Max Hospital, Noida and Ex. Senior Resident Rheumatology, AIIMS, Delhi, India
Received: October 12, 2017; Accepted: January 02, 2018; Published: January 09, 2018
Abstract
Background: Osteoarthritis is a chronic degenerative disorder of multifactorial etiology characterized by the loss of articular cartilage, resulting in joint pain, stiffness, swelling, and disability without any clear answer to its treatment and cure. Studies from intra-articular steroid with local anesthetic uses in osteoarthritis are rare from India.
Objective: To determine the effectiveness and safety of administering a combination of intra-articular corticosteroid and local anesthetic in Indian patients with knee osteoarthritis.
Methods: This, prospective, open-label, observational single-center pilot study was conducted at the Rheumatology Clinic of a tertiary care centre, from December 2015 to December 2016. This, prospective, open-label, observational single-center pilot study included patients (n=20) between 35-70 years of age, suffering from chronic knee pain for at least three months prior to inclusion, with a clinical or radiological diagnosis of knee osteoarthritis, dissatisfied with previous non-surgical management. Patients were administered injection methylprednisolone 80 mg (2 ml) plus lignocaine 1% (0.5 ml) intra-articularly which were followed with five scheduled visits i.e. baseline (visit 1), day 1 (visit 2), 6 weeks (visit 3), 12 weeks (visit 4), and 24 weeks (visit 5). Patients were evaluated on a Visual Analogue Scale [VAS] for pain and patient reported selfassessment questionnaire to evaluate other clinical effectiveness parameters.
Results: Mean age of the study population was 52.55+7.91 years. Majority (85%) were females. After administration of the injection, pain (as measured by the VAS scale) improved within a day and there was complete (100%) pain relief in all patients (as per subjective assessment) at week 1. The VAS score reduced from 8.90+0.968 at baseline to 6.35+1.387 on day 1 (mean reduction of 2.55+1.191) and 5.30+0.923 at week 1 (mean reduction of -3.60+1.273). For each of the clinical effectiveness parameters, a significantly greater proportion of patients showed ‘improved’ status than those who ‘worsened’ or remained the same. Seventy percent (14/20) patients reported ‘decreased’ frequency of Non- Steroidal Anti-Inflammatory Drug (NSAID) usage (p=0.0368).
Conclusion: Combination injection of intra-articular corticosteroid and local anesthetic is safe and effective in Indian patients with osteoarthritis. It achieves immediate pain relief, with effects lasting for at least 6 months and helps decrease NSAID usage in most patients.
Keywords: Osteoarthritis; Injections; Intra-articular; Anesthetics; Local; Visual Analog Scale; Anti-inflammatory agents; Non-steroidal
Introduction
Osteoarthritis is a chronic degenerative disorder of multifactorial etiology characterized by the loss of articular cartilage, resulting in joint pain, stiffness, swelling, and disability [1-3]. It is the most common joint disease worldwide and most commonly affects the knee joint [4,5]. In India, the prevalence of knee osteoarthritis is 28.7% [6]. Osteoarthritis of the knee joint is one of the foremost causes of global disability and is ranked as the 11th highest contributor to global disability along with hip osteoarthritis [7]. On account of the effects of disability, co-morbid disease, and treatment costs, osteoarthritis inflicts a tremendous economic burden. Additionally indirect costs such as loss of productivity, lost wages, and costs associated with the need for home care and child care further add to the disease burden [8].
In addition to the sizable economic burden, progressive functional disability associated with osteoarthritis substantially impacts quality of life in patients [9,10]. Hence treatment of osteoarthritis primarily aims at controlling pain, and improving functional disability and health-related quality of life [11]. The American College of Rheumatology (ACR) 2012 recommendations suggest several treatment modalities including non-pharmacological techniques like weight loss, patient education, and regular exercise and pharmacological drugs such as acetaminophen, oral and topical Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), tramadol, and intra-articular steroids, while conditionally recommending against the use of chondroitin sulfate, glucosamine, and topical capsaicin [12]. Acetaminophen, aspirin and NSAIDs, that are commonly used as pain relief medications can lead to gastric complications, ulcers, increased risk for hospitalization, adverse side effects, and death. [13] Likewise tramadol is associated with adverse effects such as constipation, dizziness, nausea, somnolence, headache etc. that limit its use [14].
Intra-articular steroids on the other hand exhibit a better longterm safety profile with no deleterious effects on the anatomical structure of the knee [15] Further, intra-articular steroids also significantly reduce osteoarthritic knee pain, stiffness and joint function [15-17], which in turn helps in improving quality of life, and delaying surgical interventions in patients with knee osteoarthritis.
Intra-articular corticosteroids are often used along with local anesthetics to treat osteoarthritis [18] probably due to the rationale that the local anesthetic component acts quickly after administration, to provide immediate pain relief, and its action may last until the corticosteroid component starts to exert its effect [19]. While some studies suggest that a combination local anesthetic/corticosteroid may have potential negative effects on intra-articular cell viability and cell metabolism, and may lead to chondrotoxicity [19-21], others support continued safe use of this combination in clinical practice [22]. Nonetheless the combination of intra-articular steroids and local anesthetics is routinely administered universally (either in the same syringe or separately) to treat osteoarthritis [18,23].
The potential advantage of rapid onset and prolonged duration of action offered (which enables instant pain relief and anti-inflammatory response) [19] by combination of intra-articular steroids and local anesthetics, as well as the controversy surrounding its safety [19- 22] makes it imperative to examine its effectiveness and safety in patients with knee osteoarthritis. However, studies exploring the effectiveness and safety of this combination are limited [21] especially in India. Therefore, this pilot study was conducted to determine the effectiveness and safety of administering a combination of intraarticular corticosteroid and local anesthetic in Indian patients with knee osteoarthritis.
Methods
This, prospective, open-label, observational single-center pilot study was conducted at the Rheumatology Clinic of the Medicine Department of Santosh Medical College and Hospital, Ghaziabad, from December 2015 to December 2016.
Patient selection
Adults between 35-70 years of age, suffering from chronic knee pain (pain score at least 3 cm on Visual Analogue Scale [VAS] for at least three months prior to inclusion, with a clinical or radiological diagnosis of knee osteoarthritis, dissatisfied with previous nonsurgical management including analgesics and other drugs were included after informed consent. Those with severe, advanced, destructive arthritis with deformity, neuropathic or septic arthritis, post-operative arthritis/artificial joint, hypersensitivity to study medications or contrast solutions, or those who had previously received an intra-articular injection (corticosteroid, hyaluronic acid preparation or other) were excluded.
Study procedures and data collection
At baseline, patients were administered injection methylprednisolone 80 mg (2 ml) plus lignocaine 1% (0.5 ml) intraarticularly under all aseptic precautions.
The duration of observation was 24 weeks with five scheduled visits i.e. baseline (visit 1), day 1 (visit 2), 6 weeks (visit 3), 12 weeks (visit 4), and 24 weeks (visit 5).
At baseline, data regarding demography, occupation, socioeconomic status, previous alternative treatment (ayurvedic, homeopathy, other), disease duration etc. was collected on case record forms. On all five visits, pain was measured on VAS (Visual analog scale) scale (0-10cm). (Figure 1)
Figure 1: Visual analog scale.
At 24 weeks, each patient was asked to fill up a patient selfassessment questionnaire indicating the status (‘improved’, ‘worsened’ or ‘same’; with respect to baseline) of ‘clinical effectiveness’ parameters such as range of motion of the joint; clinical symptoms like localized swelling, heat, and tenderness; duration of early morning stiffness; duration of post inactivity stiffness; increased selfdependence, self-esteem and overall confidence; overall mobility and presence in social gatherings; and overall well-being and lifestyle. In the same questionnaire, the patient was asked to indicate the change in frequency of NSAID usage (with respect to baseline) as ‘increased’, ‘decreased’ or ‘same’.
Throughout the study patients were encouraged to report any complications/adverse events, increased difficulty in movements, or restriction of mobility after intra-articular injection.
Endpoints and assessments
Effectiveness outcomes: Effectiveness was assessed on the basis of change in VAS score, proportion of patients with ‘improved’ clinical effectiveness parameters and proportion of patients with ‘reduced’ NSAID usage at 24 weeks.
Safety outcomes: Safety was assessed as incidence of Adverse Events (AEs), Serious Adverse Events (SAEs), and Adverse Drug Reactions (ADRs). An AE was defined as any untoward medical occurrence that did not necessarily have a causal relationship with treatment. When the attending physician, identified the AE as ‘related’, ‘cannot rule out the possible relation’, or ‘undeterminable’, the AE was considered an ADR. If the AE or ADR was severe enough (as determined by the attending physician) to cause death, a lifethreatening condition, hospitalization or prolonged hospitalization, persistent or significant disability, congenital diseases or anomalies in the next generation, or other medically important conditions, it was classified as a serious AE (SAE) or a serious ADR (SADR).
Statistical analysis
Sample size calculation: Assuming that a mean difference of 1.5 cm on the VAS between visits is a clinically significant improvement and a standard deviation (SD) of 2.25 cm with 80% power at 5% significance level, a sample size of 20 patients was required.
Statistical methods: All data recorded were summarized and analyzed using descriptive and inferential statistics. Mean, SD, and range (minimum–maximum) were provided for continuous variables. Frequency and percentage were presented for categorical variables. For statistical analyses of change in VAS scores, a longitudinal data model was applied to assess multiple repeated-measures using the MIXED procedure of the SAS system with random effect for subject (random intercept model). A fixed effects analyses was conducted to determine whether there were effects of time points (baseline [Day 0]), Day 1, week 1, week 6, week 12 and week 24 after the intraarticular injection). A 95% confidence interval (CI) was presented and tests were performed at a two sided 5% significance level. T-test were used to explore the differences between assessment time points. Data was explored for normality and satisfaction of parametric statistics, and no transformations were required. The statistical analyses were performed using SAS 9.3 (SAS Institute, Cary NC).
Results
A total of 20 patients with osteoarthritis were included; all completed the study.
Baseline characteristics
The mean+SD age of the included (n=20) patients was 52.55+7.91 years. Majority (85%; n=17) of the study population comprised females. Most patients (80%; n=16) had a disease duration of >2 years and had received previous treatment, of which about 55% (n=11) had received alternative treatment including homeopathic (30%; n=6) or ayurvedic treatment (25%; n=5). Hypertension (25%; n=5), diabetes mellitus (25%; n=5), and Vitamin D3 deficiency (25%; n=5) were the most common co-morbid conditions (Table 1).
Baseline characteristics
N=20
Age (years)
Mean (SD)
52.55 (7.91)
[Min; Max]
[40; 65]
35-40 years
1 (5)
41-50 years
9 (45)
51-60 years
6 (30)
61-70 years
4 (20)
Gender n (%)
Female
17 (85)
Male
3 (15)
Socio-economic Status n (%)
Low
4 (20)
Lower Middle
6 (30%)
Middle
5 (25)
Upper Middle
1 (5)
Higher Middle
4 (20)
Co-morbid conditions
Hypertension
5 (25)
Diabetes mellitus
5 (25)
Metabolic syndrome
1 (5)
Vitamin D3 deficiency
5 (25)
Previous alternative treatment n (%)
Ayurvedic
5 (25)
Homeopathy
6 (30)
None
9 (45)
Disease duration
<2years
4 (20)
>2 years
16 (80)
Table 1: Baseline characteristics.
Change is VAS score
Improvement in pain as measured by the VAS scale was seen from day 1 (visit 2). The VAS score reduced from 8.90+0.968 at baseline to 6.35+1.387 on day 1 (mean reduction of 2.55+1.191) and 5.30+0.923 at week 1 (mean reduction of -3.60+1.273). At week 1, there was complete (100%) pain relief in all patients as per subjective assessment. After week 1, improvement in VAS score continued in 70% patients at 6 weeks, 80% patients at 12 weeks and 70% patients at 24 weeks. At 24 weeks, total reduction is VAS score was 5.00+1.298 as compared to baseline (Table 2 and Figure 2).
Visit
VAS (cm)
Change from Baseline (cm)
Percent change from baseline (%)
N=20
N=20
Baseline
Mean (SD)
8.90 (0.968)
-
-
[Min; Max]
[7; 10]
-
-
Day 1
Mean (SD)
6.35 (1.387)
-2.55 (1.191)
-28.75 (14.248)
[Min; Max]
[3; 8]
[-4; 0]
[-57.14; 0]
Week 1
Mean (SD)
5.30 (0.923)
-3.60 (1.273)
-39.86 (11.518)
[Min; Max]
[4; 7]
[-6; -2]
[-60; -22.22]
Week 6
Mean (SD)
4.65 (1.089)
-4.25 (1.372)
-47.30 (12.546)
[Min; Max]
[3; 7]
[-7; -2]
[-70; -25]
Week 12
Mean (SD)
4.15 (0.875)
-4.75 (1.164)
-53.07 (9.888)
[Min; Max]
[3; 6]
[-7; -3]
[-70; -33.33]
Week 24
Mean (SD)
3.90 (1.373)
-5.00 (1.298)
-56.41 (13.973)
[Min; Max]
[2; 6]
[-8; -3]
[-80; -33.33]
Abbreviations: VAS: Visual Analog Scale; SD: Standard Deviation; Min: Minimum; Max: Maximum
Table 2: Change in Visual analog scale from baseline to 24 weeks.
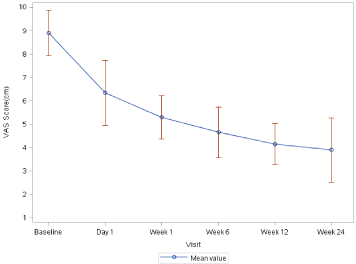
Figure 2: Change in Visual analog scale from baseline to 24 weeks.
Between 12 and 24 weeks, the VAS score worsened in 7 (35%) patients. Interestingly 5/7 patients who showed worsening of VAS score had co-morbid conditions such as hypertension and/or diabetes.
Proportion of patients with ‘improved’ self-reported clinical effectiveness parameters.
For each of the clinical effectiveness parameters (evaluated on the basis of patient self-assessment questionnaire), a significantly greater proportion of patients showed ‘improved’ status than those who ‘worsened’ or remained the same (Table 3).
Patient self-assessment
Clinical effectiveness parameters
Improved
Same
Worsened
P value for proportion of patients who improved
n (%)
n (%)
n (%)
Range of motion of joint
12 (60)
8 (40)
0
0.0339
Clinical symptoms like localized swelling, heat, and tenderness
13 (65)
7 (35
0
0.0112
Duration of early morning stiffness
13 (65)
7 (35)
0
0.0112
Duration of post inactivity stiffness
12 (60)
8 (40)
0
0.0339
Increased self-dependence, self-esteem and overall confidence
12 (60)
8 (40)
0
0.0339
Overall mobility and presence in social gatherings
12 (60)
8 (40)
0
0.0339
Overall well-being and lifestyle
12 (60)
8 (40)
0
0.03339
NSAID usage
Decreased (n%)
Same n(%)
Increased n(%)
P value for proportion of patients who decreased
13 (65)
5 (25)
2 (10)
0.0368
Abbreviations: n: Number of Patients; NSAID: Non-Steroidal Anti-Inflammatory Drugs.
Table 3: Patient self-assessment (Clinical effectiveness parameters and NSAID usage).
Proportion of patients with ‘reduced’ NSAID usage at 24 weeks.
A total of 70% (14/20) patients reported ‘decreased’ frequency of NSAID usage (p=0.0368).
Safety.
No adverse events were reported.
Discussion
In this prospective, observational single-center pilot study conducted to determine the effectiveness and safety of a combination of intra-articular corticosteroid and local anesthetic in patients with osteoarthritis, improvement in joint pain (as measured by the VAS) was observed the very next day after the injection was administered with complete pain relief within a week. In a majority of patients, the pain relief lasted up to 24 weeks after the single injection. Patients who showed a decrease in pain-relief after 12 weeks had other co-morbid conditions like hypertension and/or diabetes. Patients showing ‘improved’ clinical effectiveness parameters were significantly greater in proportion than those showing ‘worsened’ or ‘same’ status. Further a significant majority of patients reported ‘decreased’ NSAID usage in the 24 weeks after treatment.
In our study improvement in joint pain was observed within a day of initiating treatment; however complete pain relief was achieved at week 1. A systematic review and meta-analysis that included both randomized controlled trials and systematic reviews concerning use of intra-articular steroid injections in knee osteoarthritis shows both clinically and statistically (as measured by VAS) significant pain relief within one week of treatment [16]. As compared to this metaanalysis, the earlier onset of pain relief (within a day) observed in our study was probably due to the fact that none of the studies included in the meta-analysis used a local anesthetic along with the intra-articular corticosteroid to ensure that the observed pain relief was only due to the corticosteroid administration [16]. It can thus be inferred that the effect of the local anesthetic in our study led to immediate pain relief, after which the corticosteroid component took over and achieved complete pain relief at week 1. Also, unlike the above metaanalysis that shows short-lived pain relief for a maximum duration of 3-4 weeks after a single injection of intra-articular corticosteroid, in our study pain relief in most patients lasted for 24 weeks. In fact, 5/7 patients who experienced worsening of pain between 12 weeks and 24 weeks had co-morbid diabetes and/or hypertension, both of which are known to adversely impact the prognosis of osteoarthritis [24, 25].
In addition to relief in joint pain, significant number of patients reported improvement in several other clinical effectiveness parameters such as range of motion, clinical symptoms like localized swelling, heat, and tenderness, duration of early morning stiffness and duration of post inactivity stiffness, self-dependence, self-esteem and overall confidence, overall mobility and presence in social gatherings and overall well-being and lifestyle. Similarly, a randomized doubleblind study conducted at the Department of Orthopedics, TMMCRC, Moradabad between July and December 2014 shows that in addition to the beneficial effects in achieving short/long-term pain relief in knee osteoarthritis, intra-articular corticosteroids particularly methyl prednisolone (which was used in our study) provides more immediate improvement in pain, stiffness and joint function without any adverse effects [17].
On account of the deleterious digestive, renal, and cardiovascular adverse effects observed with NSAIDs usage, it is recommended that physicians prescribing NSAIDs for pain relief in osteoarthritis should consider factors such as co-morbid conditions, contraindications, and concomitant drugs [26,27] and then prescribe the lowest possible dose for the shortest required time in patients at risk of adverse effects [27,28]. A majority of patients in our study were able to reduce NSAID usage after administration of the intra-articular steroid and local anesthetic combination. Moreover, despite a shortterm beneficial effect observed with intra-articular corticosteroid administration, it can be useful to control acute exacerbations while waiting for NSAIDs to work, in patients who need rapid pain relief for an upcoming activity, where sleep is interrupted due to pain [16]. Hence a combination of intra-articular steroid and local anesthetic should be considered in patients with osteoarthritis unable to obtain relief with NSAIDs or in those experiencing gastro-intestinal or other side effects with NSAIDs [16].
There were no adverse effects of the treatment observed in our study, indicating that the drug combination was safe and well tolerated by all patients. The safety of intra-articular steroids is also supported by several other studies [15,17]. In fact the absence of major side effects is one of the primary reasons due to which intraarticular corticosteroid injections have become one of the mainstays in the management of osteoarthritis, particularly knee osteoarthritis [29].
Strengths and Limitations of the Study
This open-label, prospective, observational study was conducted in a real-world setting, and hence the study population reflects actual clinical practice. In addition to assessing improvement in joint pain in terms of VAS, the study also explores other key factors like reduction in NSAID usage after the treatment. However, this open-label study did not have a control group which could have been a source of potential bias (selection bias). Also, in this study, osteoarthritis was not evaluated by a symptom driven scale like Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). Hence further studies with larger sample size, inclusion of a control group and assessments based on additional clinical scores may be required to validate these findings. Nonetheless, this pilot study establishes the evidence for further evaluation of intra-articular corticosteroid anesthetic combination in randomized clinical trials as well as observational registries in India.
Conclusion
In conclusion, this observational study shows that a single combination injection of intra-articular corticosteroid and local anesthetic achieves immediate pain relief in patients with osteoarthritis, and the effects lasts in most patients for at least 6 months. Most patients receiving this treatment are able to decrease their NSAID usage, thus reducing its side effects, and the treatment regimen does not show any adverse effects as well. Despite certain limitations, the findings of this study lay the foundation for further large-scale randomized controlled trials that can be designed to evaluate the efficacy and safety of this combination.
Acknowledgment
The authors thank Dr. Alina Gomes for interpretation of the data as well as medical writing assistance in the development of this manuscript.
References
- Loeser RF. Age-Related Changes in the Musculoskeletal System and the Development of Osteoarthritis. Clinics in geriatric medicine. 2010; 26: 371- 386.
- Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Molecular Pain. 2007; 3: 8-8.
- Silman AJ HM. Epidemiology of the Rheumatic Diseases. 2nd edn. Oxford; Oxford University Press. 2001.
- Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133: 635-646.
- Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010; 26:355-369.
- Pal CP, Singh P, Chaturvedi S, Pruthi KK, Vij A. Epidemiology of knee osteoarthritis in India and related factors. Indian Journal of Orthopaedics. 2016; 50: 518-522.
- Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Annals of the Rheumatic Diseases. 2014; 73: 1323- 1330.
- Bitton R. The economic burden of osteoarthritis. Am J Manag Care. 2009; 15: S230-235.
- Rosemann T, Laux G, Kuehlein T. Osteoarthritis and functional disability: results of a cross sectional study among primary care patients in Germany. BMC Musculoskeletal Disorders. 2007; 8: 79.
- Song J, Chang RW, Dunlop DD. Population impact of arthritis on disability in older adults. Arthritis Rheum. 2006; 55: 248-255.
- Smink AJ, van den Ende CH, Vliet Vlieland TP, Swierstra BA, Kortland JH, Bijlsma JW, et al. Beating osteoARThritis”: development of a stepped care strategy to optimize utilization and timing of non-surgical treatment modalities for patients with hip or knee osteoarthritis. Clin Rheumatol. 2011; 30:1623- 1629.
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012; 64: 465-474.
- Pinals RS. Pharmacologic treatment of osteoarthritis. Clin Ther. 1992; 14: 336-346;
- Gana TJ, Pascual ML, Fleming RR, Schein JR, Janagap CC, Xiang J, et al. Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Med Res Opin. 2006, 22:1391-1401.
- Raynauld JP, Buckland-Wright C, Ward R, Choquette D, Haraoui B, Martel- Pelletier J, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebocontrolled trial. Arthritis Rheum. 2003; 48: 370-377.
- Godwin M, Dawes M. Intra-articular steroid injections for painful knees. Systematic review with meta-analysis. Canadian Family Physician. 2004; 50: 241-248.
- Jain P, Jain SK. Comparison of Efficacy of Methylprednisolone and Triamcinolone in Osteoarthritis of the Knee: A Prospective, Randomized, Double-Blind Study. 2015; 347.
- Saunders S LS. Injection Techniques in Orthopaedic and Sports Medicine: A Practical Manual for Doctors and Physiotherapists. London, England: Churchill-Livingstone/Elsevier; 2006.
- Farkas B, Kvell K, Czömpöly T, Illés T, Bárdos T. Increased Chondrocyte Death after Steroid and Local Anesthetic Combination. Clin Orthop Relat Res. 2010; 468: 3112-3120.
- Braun HJ, Wilcox-Fogel N, Kim HJ, Pouliot MA, Harris AH, Dragoo JL. The effect of local anesthetic and corticosteroid combinations on chondrocyte viability. Knee Surg Sports Traumatol Arthrosc. 2012, 20:1689-1695.
- Sherman SL, James C, Stoker AM, Cook CR, Khazai RS, Flood DL, et al. In vivo toxicity of local anesthetics and corticosteroids on chondrocyte and synoviocyte viability and metabolism. Cartilage 2015:1947603515571001.
- Shah K, Watson D, Campbell C, Meek RM. Intra-articular injection composed of steroid, iohexol and local anaesthetic: is it stable? Br J Radiol. 2009; 82:109-111.
- MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable Corticosteroid and Local Anesthetic Preparations: A Review for Radiologists. Radiology. 2009; 252: 647-661.
- King KB, Rosenthal AK. The adverse effects of diabetes on osteoarthritis: update on clinical evidence and molecular mechanisms. Osteoarthritis Cartilage. 2015; 23: 841-850.
- Yang W, Chan PB, Wen C, Yan CH, Chiu K. Subchondral bone disturbance in advanced knee osteoarthritis is worsened with co-morbid hypertension: does endothelin-1 matter?. Osteoarthritis and Cartilage. 24: S390.
- Dieu-Donné O, Théodore O, Joëlle ZT, Pierre D, Smaïla O, Christian C, et al. An Open Randomized Trial Comparing the Effects of Oral NSAIDs Versus Steroid Intra-Articular Infiltration in Congestive Osteoarthritis of the Knee. Open Rheumatol. 2016. 10: 8-12.
- Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013; 15: S2.
- Pelletier J-P, Martel-Pelletier J, Rannou F, Cooper C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016; 45: S22-S27.
- Uthman I, Raynauld JP, Haraoui B. Intra-articular therapy in osteoarthritis. Postgrad Med J. 2003; 79: 449-453.