
Research Article
Austin J Orthopade & Rheumatol. 2019; 6(1): 1075.
Stand-Alone Biomimetic Supple Artificial Intervertebral Discs Composed of Cubic Triaxial Three-Dimensional Fabrics
Shikinami Y*
Shikinami Yasuo Institute LLC, Japan
*Corresponding author: Shikinami Yasuo, Shikinami Yasuo Institute LLC, Japan
Received: July 23, 2019; Accepted: August 29, 2019; Published: September 05, 2019
Abstract
Objective: Existing Artificial Discs (AD) to preserve original mobility of Biological Intervertebral Disc (BID) essentially consist of the superposition of solid plates and core materials, however they are unable to fully make up its movable faculty. This article is to provide a biomimetic supple Artificial Intervertebral Disc (AID) that fulfills the mobile function quite similar to BID, which is well supplied with long-term dynamic mechanical durability and stand-alone possibility along with less invasive insertion into the correct disc site required.
Study Design: The AID system was created on the basis of our original biomimetic concept, which is substantially composed of a cubic Three- Dimensional Fabric (3DF) with a triaxial (3A) X-, Y-, Z-axes fiber alignment, and then improved into the product comprising plural textural layers, which have an elemental usual flat textual intermediate layer and convex bioactive soft textual surface layers, on which bioactive hydroxyapatite particles were deposited. The tugging ropes penetrating through the AID body with the bioactive anchoring tappets at the both ends of filament were prepared to be well fixed for standingalone.
Results: It has been created that the biomimetic supple AID to exceed the mobile capability of existing AD, which can be effectively press-fitted after minimally invasive inserting and bond to the vertebral end plates, and stoodalone in the disc space with showing mobile behavior quite similar to BID.
Conclusion: The potential clinical availability of this AID system was substantiated as one particular fibrous cartilage, the AID for the next generation.
Keywords: Artificial intervertebral disc; Biomimetic; Stand-alone; Motion preservation; Minimally invasive
Introduction
This article compiles the total process to create the supple standalone biomimetic Artificial Intervertebral Disc (AID) woven by the Tri-Axial (3A), Three-Dimensional Fabric (3DF) [1-3] that should be clinically available in the next generation. Here, the AID is not the product with same concept as the AD (Artificial Disc), and the substitute for Biological Intervertebral Disc (BID) itself, nevertheless the both are construed as a synonymous term. Spinal surgeons by no means satisfy the existing ADs in the clinical outcomes especially in lumbar for the cause of solid material construction, biomechanical motion, stand-alone system and less invasive insertion, as well as simple revision surgery. The ADs generally form three separate layers consisted of the super-positional construction with a spherical (ball or oval) core and sockets or troughs engraved on two separated plates and slide two-dimensionally on the curvature surface while contacting to the core, but cannot deform compressively due to rigidity of solid metal and plastic components [4-6], which are markedly dissimilar to BID and the disadvantages were examined in the previous works [7-10].
The BID [11,12] have a monolithic fibrous structure that mainly comprises a collagenous slant ring fiber component with lower physical strength than constituents of Solid Artificial Discs (SADs). The biological vertebral segment has limitless numbers of central axes to various normal physiological motions, and passively deforms three dimensionally responding to simultaneous or independent external loadings, along with a limitless number of central axes for distortion of the annulus including the jelly nucleus. Namely the BID deforms three-dimensionally along with the multi-axes for distortion while receiving different loadings from various direction while following simultaneously to the plural complex loadings combined together vertical compression, lateral bending, torsional twisting, dorsally flexion, ventrally extension and so, on.
The physical endurance of supple BIDs with low strength materials is achieved through support from the surrounding ligaments and musculoskeletal regions as well as the shared loading by each consecutive BID. It might be impossible that ADs made of each component with excessively high physical strengths like those of existing SADs could provide the same efficient mobility as supple BIDs. When a BID is compressed, the height naturally reduces in proportion to the compressive loading and recovers to the normal state after unloading due to firmly bonding with the endplates sticking to the Vertebral Body (VB). In other word, another effect as the BID material is due to the damping behavior for adsorbing the external stress energy that is displayed by the downward convex ‘J’-shaped stress-strain (S-S) hysteresis-loss loop, but not the upward convex ‘S’ shaped S-S one like solid materials [3,13,14].
This article elucidates every solution for an optimal AID according to procedures as itemized below 1.
First, it must be composed of biologically safe materials with good biocompatibility, which causes no serious tissue reactions arise from the base components or secondary debris generated by usual movement during the lifespan. The AIDs should be constructed by known biomaterials that have been used for a long term in vivo for humans.
Second, biomimetic constructional materials and shape should be applied to replicate the biomechanical yielding behavior with ‘J’-shaped S-S curves of normal BIDs, and to allow translation and rotation in all three planes of independent or dependent complex motion along the x, y, and z axes. This will achieve 3D tunable deforming without specific fixed pivots as in BIDs, which naturally deform receiving complex and dependent external loadings, but not an independent loading only. Therefore, it is possible to response to complex and dependent translation and rotation, coincidental plural loadings with compression, flexion, extension, or lateral bending and axial rotation, and so on, and would bring to the less potential for low back pain than the existing ADs.
Third, it must display sufficient mechanical properties, biomimetic mobility, and superb endurance with little reducing fatigue resistance while restoring to the original height; mechanical testing over 100 million biomimetic repetitive motions to equate with a 40 or 50 year lifespan would be a typical design criterion.
Fourth, several designs with size variation and deformable shape by external various loadings need to be produced in order to allow accurate placement, the ‘sweet spot’ where the implant is positioned at the correct height and depth within the disc space. Therefore, it should be a soft material to be contoured by press–fitting to the disc body inner-surface geometry; even if it would not be the lordotic shape of AID, but the convex one; this will allow the AID to be applied in cases with disc heights, spaces, and geometries that vary according to differences in patients and indications.
Fifth, the reliable stand-alone system, which can adjust to the disc space with tight press-fitting and firm bone bonding via the end plate stuck to VB, is essential, and the AID has to show osteological bioactivity on the surface to bond to disc bone.
Sixth, the AID should be set easily into the disc space by minimally invasive inserting into disc space without over distracting to endow over compression to adjoining IDs and to fix firmly with VBs and be devised with no using fixation pins or plates that can suppress original motion of an AID and break into hard debris to bring about physical irritation. In other words, the practical AID should be designed into a supple monolithic device similar to BID.
Seventh, it is provable that natural bone deposit and produce the bridging between VBs to miss the movability of AID, especially in the lumbar. Therefore, bioinert biomaterials on which bone tissues never deposit should be used essentially.
Materials, Methods and Results
Weaving method [1]
Binding up thin multi-filaments into a thick monofilament is to bring about superior flexibility than a thick monofilament with same diameter as a multifilament bound up. The respective figure and photograph as shown in due order solved every indispensable criterion to really materialize the clinical available 3DF AIDs. The construction of a high strength bioinert filament to weave the 3DF AID and the weaving method were described in previous works [3, 13,14]. Safety of the fiber materials has been already guaranteed as a biomaterial previously used in orthopedic devices and the bioinert biocompatibility doesn’t constrain hardening of AID due to bone ingrowth (ossification) into space of 3DF textures. The mobility is consequently retained as it was.
Improving on the shape adaptable to the disc space
The 3DF AID with the flat surface was selected as the first prototype (Figure 1(1)-1(4)). Thereafter they were improved to endow more flexible concave surface layers. Thereby, they could coincide with the surface geometry of reduced VB after excising BID affected. The softer concave surface layer was woven by thin filaments than the normal core layer woven by thick ones in order to easily apply to the irregular surface geometry of reduced VB (Figure 1(5) - 1(6)).
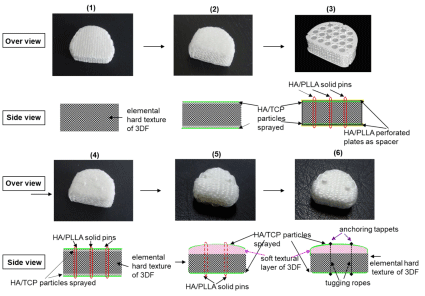
Figure 1: Improving process to create the clinically available stand-alone supple 3DF AID (6).
Confirmation of hysteresis-loss maintenance with no debris during repetitive dynamic motioning [13,14]
Lateral view change in the textural structure of total lumbar disc exhibits. As shown in Figure 2, throughout the test, textural fraying, fiber loosening in the UHMWPE filaments and PE wear-debris of the 3DF AID caused by mutual friction were not observed for 1.05x108 repetitive motions.
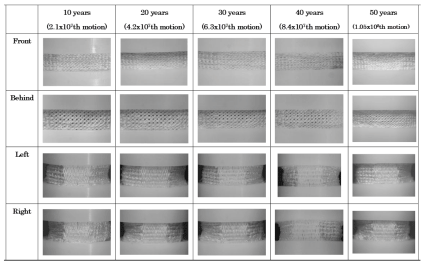
Figure 2: The side view change in elemental texture of 3DF AID in figure 1(1) during 1.05x108 repetitive passive deformation.
Modification into bioactive surface
Surface modification of UHMWPE filaments existed in the upper and lower external layer of the 3DF AID excluding the side was carried out by corona discharge oxidation to increase the hydrophilic properties of the AID (the contact angle with water was reduced from 700 to 330) and by spray treatment with bioresorbable particulate Hydroxyapatite (HA) (average particle diameter, ~ 3μm) to a depth of ~ 2 mm from the surface (density, ~20–30 g/cm3) to enhance the osteological bioactivity (osteoconductivity) (Figure 1(2)-1(6)). HA powders deposited on the surface can be observed as the radiopaque thin layer.
Preliminary animal tests [15,16]
The 3DF AID in Figure 1(2) was implanted in the disc space of sheep, which was fixed internally by bioactive, bioresorbable HA/ PLLA (Poly-L-Lactide) reinforced composite rods and metallic screws and confirmed the bonding at the vertebral end plates (Surgical technique).
The surgical techniques used were those previously described for the Acroflex lumbar disc (Depuy-Acromed, Inc., Raynham, Massachusetts, USA), which has an unrestricted sandwiched structure of titanium endplates and an olefin rubber core. Each animal underwent an anterior transperitoneal surgical procedure performed at the L5–L6 level of the lumbar spine, with total disc reconstruction. Post mortem analysis including the histopathological assessment of the systemic reticuloendothelial tissues was carried out, in addition to biomechanical multi-directional flexibility testing to determine the peak Range of Motions (ROMs) - axial rotation, flexion-extension and lateral bending-of the operative functional spine units. Quantitative histological analysis of the trabecular bone coverage at the interface between the 3DF AID and the endplates and disc bodies was also performed.
In vivo tests [17-19]
The animal tests were performed in the Institutional Animal Care and Use Committee at the Medical Biotechnology Center, University of Maryland, Baltimore, MD, and granted approval for the study of in vivo non-human primate (baboon) model. The stand–alone 3-DF Discs with pins were implanted in 38 intervertebral discs of 22 baboons, in which they were operatively processed in six baboons’ cervical (single C5-C6) after 6 months and eight baboons’ lumbar (single L5- L6. dual L3-L4) after 12 months. The durability was confirmed in all baboons’ tests without the trouble such as delaminating, dislodging, dislocating, subsiding, bridging and dispersion of frictional wear.z It would be a reason why the flexible mobility contributed to the physical fruits to deform freely in response to the various external loadings, and to enlarge a little the height due to extending force after bonding firmly to the vertebral bodies.
Summary of Baboon Tests
Biomechanical tests
Statistical analyses: All data are shown as mean ± one standard deviation and statistical analyses using a One-Way Analysis of Variance with Student-Neuman-Keuls test to determine differences between groups. Statistical results at p‹0.05 will be considered significant.
(Cervical)
Axial rotation testing demonstrated the greatest range of motion for the intact condition, which was statistically higher than the operative functional spinal units (p‹0.05). The neutral zone levels were also higher for the intact values versus the 3DF cervical device (p‹0.05).
Flexion-Extension
Flexion-extension loading highlighted the greatest similarities in range of motion values and neutral zones between the two treatment groups. There were no statistical differences in either of these two measurements when comparing the intact spine condition to the 3DF Cervical device (p›0.05).
Lateral Bending
In similarity to axial rotation, the intact spine produced greater segmental range of motion compared to the 3DF Cervical device (p‹0.05). Neutral zone levels were also higher for the intact spine versus surgical treatments (p‹0.05).
(Lumber)
Axial Rotation
Axial rotation testing of the operative functional spinal units demonstrated no statistical differences in range of segmental range or neutral zones between the single (L5-L6), dual (L3-L4) 3DF devices or intact spine condition (p›0.05).
Flexion-Extension
Flexion-extension loading highlighted the greatest differences in range of motion between the various treatment groups. The intact spine indicated a significantly greater range of motion than the dual (L3-L4) 3DF Device treatment (p‹0.05). No significant differences were observed in the neutral zone values (p›0.05).
Lateral Bending
Under lateral bending conditions, the intact spine indicated a significantly higher range in segmental motion and neutral zone compared to the remaining treatments (p‹0.05). No other significant differences were observed.
Gross Morphology Summary
All operative cervical motion segments were examined for gross evidence of fusion and histopathologic response at the time of necropsy. There was no apparent infection in any of the operative or adjacent motion segments. Based on gross examination at the time of necropsy, the systemic and reticuloendothelial tissues from all animals were considered unremarkable and without significant histopathologic changes.
Radiological Summary
Plain film radiographic analysis demonstrated no incidence of disc migration or subsidence in all cases from the six- and twelvemonth time intervals. In similarity to the lumbar device, the extent of radiolucent lines and osseointegration at the prosthesis-bone interface are not discernable on plain films as the 3DF AID device itself is radiolucent, therefore, the defining interface lines are unclear. These parameters are best addressed using undecalcified bone histology and histologic microradiographs. In the lumbar study and what has been observed in the clinical setting, the causative factors for the observed heterotopic ossification are a result of the anterior discectomy procedure and burring of the vertebral endplates. The systemic tissue histopathology in cervical and lumber application was summarized as follows. Histologic analysis of the local and systemic tissues at the six-month time (in cervical) and six- and twelve-month time (in Lumber) intervals indicated no significant pathologic changes induced by the 3DF AID treatments. The local and spinal cord specimens obtained from the operative levels -C5- C6-, - L3-L4 and L5-L6 - indicated normal histopathology with only mild infiltration of mononuclear cells, which was considered secondary to the healing response at these time intervals. These observations of macrophage activity are considered secondary to and are consistent with the healing response at the operative sites. There was no incidence of spinal cord lesions, polymorph nuclear, giant cell activity, demyelination or other significant neuro histopathological changes. Otherwise, all other systemic tissues were unremarkable and the safety of this device is secured in all respects. Tissue reaction in internal organs by HE (hematoxylin and eosin) stain is shown in Figure 3. We were generally satisfied with the results of Figure 1(5), however, there remained still concern that the solid pins for standing alone control partly the original movement of 3DF AID and bring to compression buckling of the device body so that inevitally break off itself during repetitive deforming. Additionally, there is concern to cause damage to the VBs or adjacent VBs arising from forcible insertion with a bit high distance for distracting due to protruding pin. Further improvement process to complete clinically available 3DF AID is expounded hereafter. It is provable that modification has been fully carried out as the final product shown in Figure 1(6) [1]. The 3DF AIDs are consisted of two (three) different textural layers, which have a usual textual layer at the bottom (intermediate) and convex bioactive soft textual the top (bottom) layer(s) that achieve well press fitting to the vertebral endplates. The intermediate layer is remained as the elemental 3DF AID was. The soft layer was woven by tying together another filament with reducing filament thickness or textile minuteness. The tugging UHMWPE ropes penetrating through the AID body and the dots (tappets) anchoring at the both ends of a filament are prepared to be set and fixed in the disc space as shown in Figure 4. The tappets made of bioceramics (Hydroxyapatite, Tricalcium Phosphate, Octacalcium Phosphate, AW ceramic, Bioglass® Zirconia etc.) are mounted on the upper and lower surface and connected with a tagging UHMWPE filament (rope) penetrating through the 3DF AID body vertically under tension. The length of dot is preferable 0.5~3.0mm and the thickness (diameter) is 1.0 ~ 3.0mm. The protruded length is was preferable to set tightly into the holes punched in the VB, which was appropriated from the stability of protruded pin height of Figure 3(4), 3(5). The stopper is created by melting and solidifying the ends of filament penetrating through the hole made in dots. The number of tugging rope a device is preferable three in the cervical and five in the lumber at most. When the AID is inserted into and fixed to a vertebral space, which was reduced by curettage of a damaged BID, the holes are firstly created by the puncher in the endplate surfaces contacting to the upper and lower VBs. In order to accelerate the bonding behavior at the interface between the endplates of the VBs and the 3DF AID, a slightly thicker 3DFAID should be inserted into the intervertebral space and should be in the closely attached state by press fitting. The soft layer is compressed reflectively when the load of 70 or 80 N (Newton) is applied to the cervical 3DF AID, and better press fitting to the surface being uneven is achieved effectively. This give well initial contact and bone bonding with the vertebral body endplate without the clearance between the vertebral body endplate and the 3DF AID surface. Press fitting behavior to the vertebral surface was confirmed. The AID, Figure 1(2)-1(5) can be inserted into the disc space in a similar way as SADs with keel or teeth on the plates.
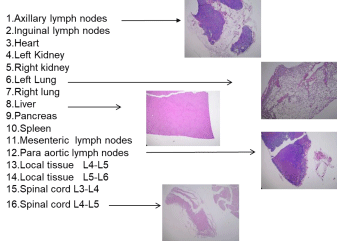
Figure 3: Examples of tissue morphology of various internal organs by hematoxylin and eosin staining.
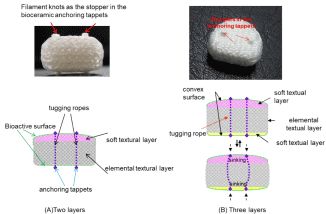
Figure 4: The textural layers of monolithic 3DF AID in figure 1(6).
The tugging ropes having filament knots as the stopper in the perforate bioceramic anchoring appets penetrate through the 3DF AID body to behave standing alone
and original flexibility of the supple 3DF AID.
The 3DF AID (Figure 1(3)-1(5)) with solid pins is conventionally inserted by the following procedure.
1. Excision of affected BID.
2. Disc height is spread by a Caspar pin in the distracter.
3. Measure the height and depth of disc space by a trial.
4. Holes to set pins or anchors for standing alone are perforated by the endplate puncher.
5. The catalyst including the 3DF AID is inserted into the disc space.
6. The blade retractor tabs of catalyst are push on disc bodies and then the insertion blade is pull off.
7. The distracter is removed and superior, inferior disc bodies get press-fitting condition with 3DF AID.
8. The catalyst is taken off and Caspar pins are also removed to complete stand-alone setting of the disc.
In case of 3DF AID, Figure 2(6), the tappets are compressed to easily sink into the 3DF AID surface with thin plane blades without the dent channel, thereafter stick out and stand-alone firmly at the holes punched in the VB. It is preferable that surgeons should insert to set the 3DF AID, which is a little thicker than the height of original disc space and the length of tugging rope under tension between both dots is equivalent or slightly short to the thickness. Therewith the 3DF AID is inserted smoothly with less obstruction of dots protruding on the surface. The inserting jigs may be unnecessary in some cases. Thereafter, the dots surely stick out and the 3DF AID stands alone firmly at the hole punched in VB while compressively contracting by the weight of head or bust, and be able to follow a bit extension of disc space height. Even in case that the dots protrude efficiently on the surface, in which the ropes with the same height as original disc space vertically penetrate through, the 3DF AID slides to inserts while anchoring dots are sinking into the top and bottom under compressive contracting as shown in Figure 5 and Figure 6 which substantiates less invasive insertion. Figure 7(1) shows mobile behavior in response to deforming of the disc space.
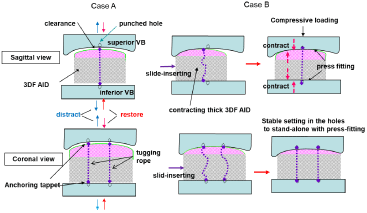
Figure 5: Two cases of insertion into the disc space of the 3DF AID shown in figure 1(6) (Figure 4(A)).
Case A shows insertion into the disc space distracted. Case B shows insertion into the normal disc space undistracted. In case B, slide-inserting is performed
when the tappets at the ends of a pliable tugging rope sink into the surface and thereafter project on in the holes prepared in the vertebral disc to acquire stable
stand- alone setting.

Figure 6: Minimally invasive insertion model into the disc space to secure the condition for standing alone and press-fitting.

Figure 7 of 1: Freely passive motions in the disc space of the supple 3DF AID shown in figure 1(6) (Figure 4(A)).
The 3DF AID embedded in the disc space transform freely along with various spinal motions. The tugging rope has no control the passive deformation due to compressing, flexing-extending, bending, twisting, and their simultaneous complex motioning and hold original mobility, which is far superior to the case using solid pins. Figure 7(2) shows an example of compressive deforming with lateral bending. The flexibility of 3DFAID texture changes in the shape conforming coincidental plural loadings such as compressing, flexing, extending, lateral bending and axial distortion, which is quite similar to BID and would probably bring to much less potential for low back pain than the existing SADs.
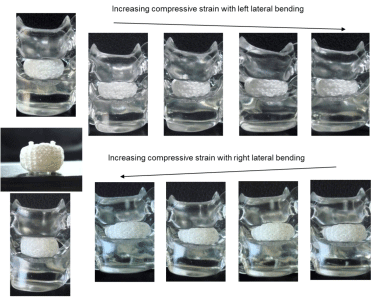
Figure 7 of 2: complex deforming models showing lateral bending with compressive loading.
The UHMWPE filament penetrated through the hole of radiopaque zirconia beads as the markers, which were observed by the C-arm radiographic imaging scanner intensifier; therewith the ROM values are estimated. Figure 8 shows Cervical C-arm image of 3DF AID shown in Figure 1(5). The image with complex moving fully proves the flexibility of 3DF AID even in this case, because the distance and the slope between markers is variable depending upon the segment implanted in, though the AIDs are unused ordinarily in plural segments at once. It is more effective in 3DF AID shown in Figure 1(6).
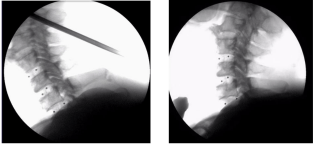
Figure 8: Cervical C-arm image of human cadaver obtained at St. Maria
catholic Univ. in seoul, Korea.
Discussion
It is to the point that the existing ADs for disc replacement arthroplasty preserve the motion of being unlike or dissimilar to BID, and still now being at the trial stage waiting for the ideal devices for total disc replacement. Those movable ADs are constructed by both portions of Intervertebral Disc (ID) and disc body as AD prosthesis. The projections on the plates are integrated with the disc body, and the spherical core with function of ID separates from plates to play a part in the disc body to slide on each plate surface without compressive deforming. The clinical outcomes of ADs were not as satisfying as surgeons expected and received reputation as unpopular devices. The ADs are insufficient to preserve the same motion of BID and very difficult to sweep away perfectly some clinical complication affected. The AIDs developed here are quite similar devices and possess monolithic fibrous construction to BID that freely shows passive deforming to the tree-dimensional directions that realize the natural motion of BID itself. The stand-alone biomimetic 3DF AID has unique concept that is quite different from ADs in material, construction, deformable principle, and durability as well as stand-alone system with less invasive insertion, so that dispels all of disadvantages of the existing ADs. We have continued improving the 3DF AID for many years that have completed a system and just desire earnestly the clinical trial for humans. We can produce 3DF AIDs with various sizes and shapes as surgeons require. At present, surgeons often use the ligamentotaxis method that is an operative procedure for lateral approach to the lumber spine without desmotomy, which is effective for minimally invasive insertion of large size of undeformable solid cages or spacers to acquire stand-alone stability [20]. We can produce the similar shape and size of supple 3DF AID by permuting the numbers of filaments in the warps (v-axis) and wefts (w-axis) with respect to the basis x (y-axis). Internal or external fixation devices are unused ordinary; however, the devices may be removed after surely bonding with endplates or VB as the standardization. The BID is a kind of fibro, elastic cartilage. It is surely thought that the tough and supple 3DFs also provides the potential scaffold for regenerating cartilaginous tissue or substitutes a joint cartilage itself, to which dynamic mechanical stress loads just after implanting along with an individual moving.
Conclusion
There used generally two sorts of devices for clinical spinal fusion. Many kinds of solid cages have been devised for spinal interbody fusion to fix firmly disc bodies with affected ID, which is said to relieve the pain with relatively high success ratio. The stand-alone cages have to be endowed osteoconductivity to bond at the interface between the cage and the VB. However, it is inevitable that the fusion site loses its mobility so that transfers excessive stresses to the adjoining discs and remarkably suppress the ROM value. Surgeons have been waiting for developing of the movable AID to preserve natural motion of BID itself. It is said again that the existing ADs are not the real AID that has equivalent function to BID. The supple, movable 3DF AID as specifically mentioned in this article has dissolved various criterial problems. Unfortunately, the 3DF AID is supposed to be a same device as the existing ADs. It is a natural course pursued when a medical product was developed unsuccessfully in clinical stage would lose surgeon’s confidence. Even in the quite unique material and system to be used clinically in the same medical field, it cannot break easily out the sludge due to being regarded as a same kind of product and system. This AID also is now in the similar critical condition. This might be caused by misunderstanding on both technical terms, AD and AID. It requires a lot of time, much labor, high cost and great project activity involving surgeons, hospitals, companies as well as universities before reaching the stage to be used clinically for humans. This might be an extremely high obstruction and never be an independent, stand-alone work. We believe deeply the 3DF AID would surely response to surgeons’ and patients’ eager expectation, and will greatly benefit patients suffering from serious disc disorders in the near future.
Acknowledgement
I really appreciate all of the medical doctors and biomaterial scientists who have engaged on this project to research and develop this AID for more than 15 years.
The Foundation of Advanced Technology Initiative for New Industry Creation of Japan Supported. The Institutional Animal Care and Use Committee at the Medical Biotechnology Center, University of Maryland, Baltimore, MD, granted approval for the study of in vivo non-human primate model.
FDA and MHLW Japan device/drug status: not applicable.
References
- Shikinami Y, Kawabe Y, Yasukawa K, Tsuta K, Kotani Y, Abumi K, et al. Standalone Biomimetic Artificial Intervertebral Disc System. 2014; US8690947B2.
- Shikinami Y, Biocompatible Implant Material Composing Tri-axial or More Three-dimensional Fabric. Jan. 27, 1998; US005711960A.
- Shikinami Y, Kawarada H. Potential application of a three-dimensional fabric (3-DF) as an implant. Biomaterials. 1998; 19: 617-635.
- Engelthardt S, Shirley A. Orthopaedic product news September/October. The landscape for spinal products in the U.S.: lots of activity as battle for market share continues. OH: Knowledge Enterprises Inc. 2007; 32-40.
- Guyer RD, McAfee PC, Banco RJ, Stephen H, Hochschuler, Fabian D, et al. Prospective, randomized, multicenter food and drug administration investigational device exemption study of lumbar total disc replacement with Charité artificial disc versus lumbar fusion: five-year follow-up. The Spine J. 2009; 9: 374-386.
- Punt IM, Visser VM, Van Ooji, Ilona M, PuntViolette M, Willem H. Schurink, et al. Complications and reoperations of the SB Charité lumbar disc prosthesis: experience in 75 pts. Eur Spine J. 2008; 17: 36-43.
- Punt. I, Marc Rijsbergen, Keita Ito, ItoLodewijk van, RhijnAndré van, OoijPaul Willems, et al, Subsidence of SB Charité total disc replacement and the role of undersizing. Eur Spine J. 2013; 22: 2264-2270.
- Anderson PA, Rouleau JP. Intervertebral disc arthroplasty. Spine 2004; 29:2779-2786.
- Bao QB, McCullen GM, Higham PA, Dumbleton JH, Yuan HA. The artificial disc: theory, design and materials. Biomaterials 1996; 17: 1157-1167
- Hallab N, Link HD, McAfee PC. Biomaterial optimization in total disc arthroplasty. Spine 2003; 28: S139–152.
- Humzah MD, Soames RW. Human intervertebral disc: structure and function. Anat Rec. 1988; 220: 337-356.
- H. Inoue, T. Takeda, Three-Dimensional Observation of Collagen Framework of Lumbar Intervertebral Discs Acta Orthop.Scand. 1975; 46: 949
- Shikinami Y, Kotani Y, Cunningham BW, K. Abumi, K Kaneda. A biomimetic artificial disc with improved mechanical properties compared to biological intervertebral discs. Adv. Funct Mater. 2004; 14: 1039-1046.
- Shikinami Y, Kawabe K, Y, Yasukawa K, Tsuta K, Kotani Y, Abumi K. A biomimetic artificial intervertebral disc system composed of a cubic threedimensional fabric. The Spine Journal. 2010; 10: 141-152.
- Kadoya K, Kotani Y, Shikinami y, Norimichi Shimamoto, Tsuyoshi Kadosawa, Kiyoshi Kaneda, et al. Biomechanical and Morphologic Evaluation of a Threedimensional Fabric Sheep Artificial Intervertebral Disc. In Vitro and In Vivo Analysis SPINE. 2001; 26: 1562-1569
- Takahata M, Kotani Y, Shikinami Y, Norimichi Shimamoto, Tsuyoshi Kadosawa, Kiyoshi Kaneda. et al: Bone Ingrowth Fixation of Artificial Intervertebral Disc Consisting of Bioceramic-Coated Three-dimensional Fabric SPINE. 2003; 28: 637-644.
- Kotani Y, Abumi K, Shikinami Y, Norimichi Shimamoto, Tsuyoshi Kadosawa, Kiyoshi Kaneda. et al: Artificial Intervertebral Disc Replacement Using Bioactive Three-dimensional Fabric. Design, Development, and Preliminary Animal Study. SPINE. 2002; 27: 929-936
- Kotani Y, Abumi K, Shikinami Y, Ken Kadoya, Tsuyoshi Kadosawa, Akio Minami, et al: Two years observation of artificial intervertebral disc replacement: results after supplemental ultra-high strength bioresorbable spinal stabilization. J Neurosurg. Spine. 2004; 100: 337-342
- Kotani Y, Cunningham BW, Abumi K, Niabin Hu, Manabu ItoYasuo, Shikinami, et al. Multidirectional flexibility analysis of anterior and posterior lumbar artificial disc reconstruction: in vitro human cadaveric spine model. Eur Spine J. 2006; 15: 1511-1520
- Marchi L, Abdala N, Oliveira L, Rodrigo Amaral, Etevaldo Coutinho, Luiz Pimenta, et al. Stand-Alone Lateral Interbody Fusion for the Treatment of Low-Grade Degenerative Spondylolisthesis. Scientific World Journal 2012; 2012: 456346.