
Research Article
Austin J Orthopade & Rheumatol. 2019; 6(2): 1081.
Characterization of Femoral Head Destruction in the Early Stage of Rapidly Progressive Osteoarthritis of the Hip
Yasuda T*, Matsunaga K, Hashimura T, Tsukamoto Y, Sueyoshi T, Ota S, Fujita S and Onishi E
Department of Orthopaedic Surgery, Kobe City Medical Centre General Hospital, Japan
*Corresponding author: Yasuda T, Department of Orthopaedic Surgery, Kobe City Medical Center General Hospital, Japan
Received: November 29, 2019; Accepted: December 18, 2019; Published: December 25, 2019
Abstract
Objectives: This study aimed to characterize femoral head destruction in the early stage of rapidly progressive osteoarthritis of the hip (RPOH) and clarify its association with potential pathological factors of RPOH.
Methods: This study included 25 female patients with RPOH diagnosed using a series of radiographs and computed tomography, which demonstrated chondrolysis ›2 mm with femoral head destruction during 12 months from the disease onset. The extent of femoral head destruction was determined on radiographs. Cortical thickness index (CTI), pelvic tilt, and serum concentrations of matrix metalloproteinase-3 (MMP-3) were analyzed.
Results: Femoral head destruction of RPOH within 12 months from the onset were classified into two types based on the extent of destruction. Whereas partial destruction showed ‹20% collapse ratio, massive destruction demonstrated ›40% collapse ratio. Increased posterior pelvic tilt was found in massive destruction. Femoral head destruction started earlier within the first 6 months in massive destruction compared with that in partial destruction. From receiver operating characteristic curve analysis, pelvic tilt differentiated the femoral head destruction types using the initial radiograph at the onset before first demonstration of femoral head destruction. No difference was found in CTI or MMP-3 between the two types.
Conclusion: Femoral head destruction of RPOH during 12 months after the onset was classified into massive and partial destructions based on the extent of destruction. Increased posterior pelvic tilt may work as a mechanical factor. Pelvic tilt could predict the extent of femoral head destruction in RPOH at the time before the initiation of bone destruction.
Keywords: Bone destruction; Classification; Diagnosis; Hip joint; Pelvic tilt; Rapidly progressive osteoarthritis
Abbreviations
CT: Computed Tomography; CTI: Cortical Thickness Index; MMP: Matrix Metalloproteinase; OH: Osteoarthritis of the Hip; RPOH: Rapidly Progressive Osteoarthritis of the Hip; ROC: Receiver Operating Characteristic; SD: Standard Deviation
Introduction
Rapidly progressive osteoarthritis of the hip (RPOH) is an unusual subset of osteoarthritis of the hip (OH) characterized by rapid chondrolysis with progressive loss of the joint space as the first manifestation of the disease. The standard definition of RPOH is chondrolysis ›2mm in 1 year or 50% joint space narrowing in 1 year [1]. RPOH occurs mostly in elderly women and causes severe disability [2]. Because rapid progression of RPOH makes it difficult to obtain sequential radiographs in its early stage [3], the process of disease progression in the early stage remains unclear. Based on the periodic radiologic findings from the onset of the disease in previous studies [3-5], however, RPOH progression could be classified into several stages. In some hips with RPOH, rapid joint space narrowing is observed without femoral head or acetabular bone loss during the first 12 months. In other hips with RPOH, subsequent to rapid joint space narrowing, the femoral head and acetabulum are destroyed within 6-12 months after initial presentation. In RPOH with bone destruction, delayed treatment may result in poor outcome with considerable difficulties in total hip arthroplasty because of severe loss of bone stock in combination with increased blood loss during surgery [6,7]. Therefore, there is a need for early diagnosis of RPOH before the occurrence of significant bone destruction.
Although the pathogenesis of RPOH is still unclarified, several pathological conditions have been suggested as the potential causes of RPOH. Subchondral insufficiency fracture of the femoral head resulting from osteoporosis could lead to RPOH [8,9]. Pelvic posterior inclination in RPOH has been shown to be greater compared with that in OH, which may play a role in development of RPOH as a mechanical factor [10]. Serum levels of matrix metalloproteinase (MMP)-3 are found to be increased in patients with RPOH than those with OH [11]. MMP-3 is likely to work in cartilage degradation of RPOH because the essential action of MMP-3 in joint destruction is in the cartilage [12]. Currently, there is no information about association between those potential causes and the disease progression of RPOH in the early stage.
This study aimed to characterize the process of bone destruction in RPOH by sequential radiological data in its early stage and investigate its association with the proposed pathological factors, MMP-3, pelvic tilt, and osteoporosis.
Methods
Patients and their demographic, radiographical, and hematological data
This monocentric retrospective study was approved by the Ethics Committee of Kobe City Medical Center General Hospital (the acknowledgement number: k190516). Informed consent was not received due to the retrospective nature of the study. This study enrolled female patients with sufficient clinical records including the onset of hip pain, age and body mass index (BMI) at the onset, a series of radiographs at regular intervals of 2-3 months during the period of ›12 months from the onset of hip pain, and hematological data including MMP-3. Serum samples were collected by venous puncture from each patient at the first visit to our hospital. Because every patient was referred to our hospital by local clinics, durations between the onset of hip pain and blood test were different in different patients. Serum concentration of MMP-3 was determined by latex turbidimetric immunoassay. Male patients were excluded because RPOH occurs mainly in elderly females and the reference intervals of MMP-3 are different between males (36.9-121 ng/ml) and females (17.3-59.7 ng/ml). According to a survey over a consecutive series of patients with hip pain from 2012 through 2018, the hip joints of 25 patients met the diagnostic criteria of RPOH; chondrolysis ›2 mm in 1 year [1] and developed femoral head destruction within 1 year from the onset of hip pain. In each case, the disease was unilateral without evidence of antecedent OA, osteonecrosis, neuropathy, infection, or inflammatory disease including rheumatoid arthritis.
Radiological parameters
The cortical thickness index (CTI) was calculated as the ratio of the femoral diaphyseal diameter minus the intramedullary canal diameter to the femoral diaphyseal diameter [13]. These diameters were measured 10 cm below the midpoint of the lesser trochanter. There is significant correlation between CTI and bone mineral density of the hip [14,15]. Pelvic tilt was estimated by the ratio between the vertical and the horizontal diameters of the pelvic foramen on the supine anteroposterior radiograph [16]. These parameters were measured on the initial radiograph at the onset of hip pain using a PACS (picture archiving and communication system) workstation. On the last radiograph taken within 12 months after the onset of hip pain, the vertical distance was measured using PACS between the two separate lines parallel to the radiographic teardrop line drown through the most proximal and distal portions of the femoral head. Femoral head collapse ratio that indicates the extent of femoral head collapse was calculated with the method as shown in Figure 1. Computed tomography (CT) was used to evaluate bone destruction in the hip joint.
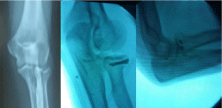
Figure 1: Femoral head collapse ratio. On the last anteroposterior radiograph
taken within 12 months after the onset of hip pain, the vertical distance is
measured between the two separate lines parallel to the radiographic
teardrop line drown through the most proximal and distal portions of the
affected (A) and non-affected (B) femoral heads. Femoral head collapse ratio
(%) is calculated with the equation as indicated.
Statistical analysis
The data are expressed as the mean ± standard deviation (SD). Student’s t test was performed for continuous normally distributed data and the Mann-Whitney U test was performed for non-normally distributed data. Differences between groups were also compared using Fisher’s exact test. Receiver operating characteristic (ROC) curve analysis was performed and cut-off values for high specificity or high sensitivity were identified for pelvic tilt parameters. Statistical analyses were conducted in SPSS for Windows, Version 25 (SPSS Inc., Chicago, Illinois, USA). The level of significance was set at P‹0.05.
Results
Classification of RPOH progression based on the extent of femoral head destruction in the early stage
Following rapid joint space narrowing, femoral head destruction was observed by CT within 12 months after the onset of hip pain in 25 female patients. When the femoral head collapse ratio was calculated using the last radiograph taken within 12 months after the onset of hip pain (Figure 1), the extent of femoral head destruction in those patients were classified into two distinct groups (Figure 2). Whereas 17 patients showed partial destruction of the femoral head with the femoral head collapse ratios of ‹20% (range of femoral head collapse ratios, 2.3-19.9%) (Figure 3), 8 patients demonstrated its massive destruction with those of ›40% (range of femoral head collapse ratios, 41.7-81.4%) (Figure 4). In both groups femoral head destruction started in the anterior portion of the femoral head (Figures 3 and 4). Concomitant with massive femoral head destruction, 4 of the 8 patients developed severe acetabular destruction (Figure 4).
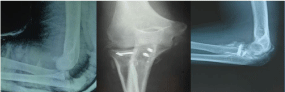
Figure 2: Box plot showing femoral head collapse ratios for partial and
massive destructions of the femoral head in patients with rapidly progressive
osteoarthritis of the hip. The top and bottom of the box represent the
interquartile range, the line within the box represents the median, and the
whiskers indicate the range.
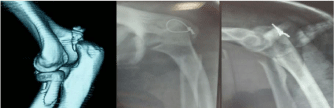
Figure 3: Partial femoral head destruction in rapidly progressive osteoarthritis
of the hip. Left hip joint showing chondrolysis without femoral head destruction
on computed tomography (CT) at 6 months after the onset. Note partial
destruction of the anterior portion in the femoral head (femoral head collapse
ratio, 11.1%) with anterior wall destruction of the acetabulum on CT at 12
months.
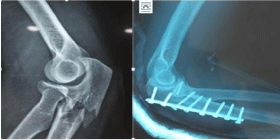
Figure 4: Massive femoral head destruction in rapidly progressive
osteoarthritis of the hip. Right hip joint showing chondrolysis with partial
destruction of the anterior portion in the femoral head on CT at 4 months after
the onset. Note disappearance of the femoral head (femoral head collapse
ratio, 81.4%) concomitant with severe acetabular destruction at 8 months
after the onset.
Comparison of radiologic and hematologic parameters between RPOH with partial and massive destruction of the femoral head (Table 1)
We further analyzed the radiologic and hematologic data between RPOH with partial and massive destructions of the femoral head within 12 months after the onset of hip pain. There was no difference in CTI or MMP-3 between the two groups. The pelvic tilt parameter significantly decreased in RPOH with massive femoral head destruction compared with the disease with partial destruction, indicating an increase in posterior pelvic tilt in patients with RPOH who developed massive destruction. The ROC curve analysis (Figure 5) showed that the area under the curve for the pelvic tilt was 0.787 (P=0.0069). Regarding the cut-off value calculated from the ROC curve, the pelvic tilt parameter demonstrated 87.5% sensitivity and 64.7% specificity with the cut-off at 0.522. Because CT was taken in each patient at 6 months and earlier after the onset of hip pain, the presence or absence of femoral head destruction by CT at 6 months were compared between the two groups (Table 2). Femoral head destruction started before the first 6 months after the onset in all patients who developed massive destruction, compared with 7 of 17 patients who developed partial destruction. In contrast to no patient with massive destruction, femoral head destruction was demonstrated by CT for the first time at 6 months and over after the onset in 10 of the 17 patients with RPOH with partial destruction. This difference was statistically significant by Fisher’s exact test (P=0.006).
Figure 5: Receiver operating characteristic (ROC) curve analysis of pelvic
tilt parameters for differentiation of massive femoral head destruction (n=8)
from partial destruction (n=17) shown by ROC curve. Pelvic tilt parameters
were determined using the initial radiograph at the onset of hip pain. The area
under the curve value is 0.787 (P=0.0069).
Discussion
The current diagnostic criteria of RPOH (chondrolysis >2 mm or 50% joint-space narrowing per year) described by Lequesne [1] need to follow the patient in evolution for 12 months. Previous case series of RPOH have mentioned that bone destruction may occur within 6-12 months after initial presentation [3-5]. Probably due to lack of sequential clinical findings in its early stage, however, the progression of bone destruction process has not been fully explored in RPOH. This retrospective review of the 25 female RPOH patients with a series of radiologic data from the onset of hip pain has demonstrated that some hips with RPOH develop bone destruction within the first 12 months from the disease onset. In addition, femoral head destruction may start before 6 months after the onset of hip pain. Based on the findings of the present study, the process of femoral head destruction could be classified into two distinct types according to the extent of femoral head destruction within 12 months after the onset of hip pain.
This study has also shown for the first time that posterior pelvic tilt is associated with the extent of femoral head destruction in RPOH patients. Recently, the potential role of spino-pelvic sagittal alignment in development of OH has received much attention. Hip-spine syndrome first described by Offierski and MacNab suggests that the hip-spine relationship may contribute to the development of both hip and spine disorders [17]. When lumbar lordosis is elevated, posterior pelvic tilt increases in order to maintain sagittal balance [18]. Thus, degenerative lumbar kyphosis may cause posterior pelvic tilt in the sagittal plane. Because the sacrum rotates around the bicoxofemoral axis as a whole unit without movement within the rigid pelvic ring [19,20], the compensatory posterior tilt of the pelvis could decrease apparent femoral head coverage [21-23] by creating a more vertical articular surface of the acetabulum [24]. A decrease in anterior acetabular coverage of the femoral head by posterior pelvic tilt results in increased stress concentration in the weight-bearing area of the hip joint without developmental dysplasia [10], and could be one of the pathological factors in the development of OH [25]. Compared with OH, female patients with RPOH exhibit lower lumbar lordosis and greater posterior pelvic tilt [26]. Collectively, initial destruction in the anterior portion of the femoral head is likely to be caused by undercovering of the anterior femoral head as a result of increased posterior pelvic tilt in the present series of patients with RPOH.
Joint contact stress is generally believed to influence development of OH. Because patients with hip dysplasia have higher contact stress than healthy subjects [27,28], insufficient acetabular coverage can concentrate the hip loads on a smaller weight-bearing surface compared with normal hips. When elevated joint contact stress at the femoral-acetabular interface and acetabular rim complex reaches a critical level, femoral head destruction could occur in some hips with RPOH. Therefore, massive destruction of the femoral head in RPOH may be a result of increased load bearing by exacerbation of insufficient acetabular coverage in combination with greater posterior tilt of the pelvis.
This study suggests that massive destruction of the femoral head is highly associated with significant destruction of the acetabulum. Because delayed treatment for such conditions may provide considerable difficulties in total hip arthroplasty, it is desirable to identify the patients who will develop massive femoral head destruction in the early stage of RPOH. MMP-3 is thought to play a critical role in rapid chondrolysis as the first manifestation of RPOH [11]. Indeed, MMP-3 increased at significantly higher levels above the reference interval (17.3-59.7 ng/ml) in the present series of patients with RPOH (Table 1), indicating that MMP-3 may be a useful marker to identify patients who will develop RPOH among patients with hip pain in the early stage. From the ROC curve analysis, posterior pelvic tilt is likely to be the determinant for differentiation of the massive destruction from the partial destruction on the initial radiograph at the onset of hip pain. A prospective study that includes a higher number of patients in the early stage of RPOH before initiation of bone destruction is needed to investigate a predictive role of pelvic tilt in combination with MMP-3 in early identification of massive femoral head destruction.
femoral head destruction
P
partial (n=17)
massive (n=8)
age (years)
71.1 ± 11.3
76.6 ± 6.7
0.211*
body mass index (kg/m2)
23.4 ± 3.1
20.8 ± 2.7
0.055*
femoral head collapse ratio (%)
7.7 ± 4.6
58.8 ± 12.1
<0.001*
cortical thickness index
0.537 ± 0.054
0.516 ± 0.070
0.424*
pelvic tilt parameter
0.534 ± 0.084
0.404 ± 0.142
0.008*
matrix metalloproteinase-3 (ng/ml)
133.9 ± 99.8
149.4 ± 137.2
0.977**
duration between the onset of hip pain and blood test (months)
6.41 ± 4.05
4.50 ± 2.33
0.409**
Values are expressed as mean ± SD. P values are determined by t test (*) and Mann-Whitney U test (**) for parametric and nonparametric tests, respectively. Values highlighted in bold indicate statistical significance (P<0.05).
Table 1: Comparison of clinical data between partial and massive femoral head destructions in rapidly progressive osteoarthritis of the hip.
duration between the onset and the first demonstration by CT of femoral head destruction
P*
< 6 months
> 6 months
partial destruction
7
10
0.006
massive destruction
8
0
CT: Computed Tomography; *Comparison by Fisher’s exact test.
Table 2: Comparison of the duration between the onset and the first demonstration of femoral head destruction by computed tomography.
There are several limitations to the present study. First, this was a retrospective study with some selection bias. Second, this study investigated a small number of female subjects with the absence of males and healthy control. However, the number of RPOH patients investigated in previous studies has ranged from 12 to 20 [11,29-31]. In addition, it may be difficult to recruit a large number of patients with a complete set of data within 12 months after disease onset. Third, the spinal and pelvic alignment was not assessed with whole-spine and lower extremity radiographs. Fourth, MMP-3 in each subject was determined at a single time point (the first visit to our hospital). Time course of MMP-3 remains unclear in each patient with RPOH during the disease progression from the onset. Fifth, we only evaluated CTI without bone mineral density measurement at the proximal femur.
In conclusion, the process of femoral destruction in RPOH during 12 months after the onset of hip pain may be classified into two types, partial and massive, based on the extent of femoral head destruction. There is possibility that pelvic tilt at the disease onset could differentiate the destruction types of RPOH at the time before the initiation of bone destruction.
Funding
This work was supported by the Japan Hip Joint Foundation.
References
- Lequesne M. Rapid Destructive Coxarthritis. Rhumatologie. 1970; 2: 51-63.
- Bock GW, Garcia A, Weisman MH, Major PA, Lyttle D, Haghighi P, et al. Rapidly Destructive Hip Disease: Clinical and Imaging Abnormalities. Radiology. 1993; 186: 461-466.
- Sugano N, Ohzono K, Nishii T, Sakai T, Haraguchi K, Yoshikawa H, et al. Early MRI Findings of Rapidly Destructive Coxopathy. Magn Reson Imaging. 2001; 19: 47-50.
- Zazgyva A, Gurzu S, Gergely I, Jung I, Roman CO, Pop TS. Clinico- Radiological Diagnosis and Grading of Rapidly Progressive Osteoarthritis of the Hip. Medicine (Baltimore). 2017; 96: e6395.
- Pivec R, Johnson AJ, Harwin SF, Mont MA. Differentiation, Diagnosis, and Treatment of Osteoarthritis, Osteonecrosis, and Rapidly Progressive Osteoarthritis. Orthopedics. 2013; 36: 118-125.
- Postel M, Kerboull M. Total Prosthetic Replacement in Rapidly Destructive Arthrosis of the Hip Joint. Clin Orthopaedics Relat Res. 1970; 72: 138-144.
- Charrois O, Kahwaji A, Vastel L, Rosencher N, Courpied JP. Blood Loss in Total Hip Arthroplasty for Rapidly Destructive Coxarthrosis. Int Orthop. 2001; 25: 22-24.
- Yamamoto T, Bullough PG. The Role of Subchondral Insufficiency Fracture in Rapid Destruction of the Hip Joint: A Preliminary Report. Arthritis Rheum. 2000; 43: 2423-2427.
- Watanabe W, Itoi E, Yamada S. Early MRI Findings of Rapidly Destructive Coxarthrosis. Skelet Radiol. 2002; 3: 35-38.
- Watanabe W, Sato K, Itoi E, Yang K, Watanabe H. Posterior Pelvic Tilt in Patients with Decreased Lumbar Lordosis Decreases Acetabular Femoral Head Covering. Orthopedics. 2002; 25: 321-324.
- Masuhara K, Nakai T, Yamaguchi K, Yamasaki S, Sasaguri Y. Significant Increases in Serum and Plasma Concentrations of Matrix Metalloproteinases 3 and 9 in Patients with Rapidly Destructive Osteoarthritis of the Hip. Arthritis Rheum. 2002; 46: 2625-2631.
- Okada Y, Nagase H, Harris ED Jr. A Metalloproteinase from Human Rheumatoid Synovial Fibroblasts that Digests Connective Tissue Matrix Components. Purification and Characterization. J Biol Chem. 1986; 261: 14245-14255.
- Yeung Y, Chiu KY, Yau WP, Tang WM, Cheung WY, Ng TP. Assessment of the Proximal Femoral Morphology Using Plain Radiograph-Can It Predict the Bone Quality? J Arthroplasty. 2006; 21: 508-513.
- Baumgärtner R, Heeren N, Quast D, Babst R, Brunner A. Is the Cortical Thickness Index a Valid Parameter to Assess Bone Mineral Density in Geriatric Patients with Hip Fractures? Arch Orthop Trauma Surg. 2015; 135: 805-810.
- Nguyen BN, Hoshino H, Togawa D, Matsuyama Y. Cortical Thickness Index of the Proximal Femur: A Radiographic Parameter for Preliminary Assessment of Bone Mineral Density and Osteoporosis Status in the Age 50 Years and over Population. Clin Orthop Surg. 2018; 10: 149-156.
- Nishihara S, Sugano N. Nishii T, Ohzono K, Yoshikawa H. Measurements of Pelvic Flexion Angle Using Three-Dimensional Computed Tomography. Clin Orthop Relat Res. 2003; 411: 140-151.
- Offierski CM, MacNab I. Hip-Spine Syndrome. Spine (Phila Pa 1976). 1983; 8: 316-321.
- Yoshimoto H, Sato S, Masuda T, Kanno T, Shundo M, Hyakumachi T, et al. Spinopelvic Alignment in Patients with Osteoarthrosis of the Hip: A Radiographic Comparison to Patients with Low Back Pain. Spine (Phila Pa 1976). 2005; 30: 1650-1657.
- Sturesson B, Uden A, Vleeming A. A Radiostereometric Analysis of Movements of the Sacroiliac Joints during the Standing Hip Flexion Test. Spine (Phila Pa 1976). 2000; 25: 364-368.
- Jackson RP, Peterson MD, McManus AC, Hales C. Compensatory Spinopelvic Balance over the Hip Axis and Better Reliability in Measuring Lordosis to the Pelvic Radius on Standing Lateral Radiographs of Adult Volunteers and Patients. Spine (Phila Pa 1976). 1998; 23: 1750-1767.
- Dandachli W, Ul Islam S, Richards R, Hall-Craggs M, Witt J. The Influence of Pelvic Tilt on Acetabular Orientation and Cover: A Three-Dimensional Computerized Tomography Analysis. Hip Int. 2013; 23: 87-92.
- Takemitsu Y, Harada Y, Iwahara T, Miyamoto M, Miyatake Y. Lumbar Degenerative Kyphosis. Clinical, Radiological and Epidemiological Studies. Spine (Phila Pa 1976). 1988; 13: 1317-1326.
- Ross JR, Nepple JJ, Philippon MJ, Kelly BT, Larson CM, Bedi A. Effect of Changes in Pelvic Tilt on Range of Motion to Impingement and Radiographic Parameters of Acetabular Morphologic Characteristics. Am J Sports Med. 2014; 42: 2402-2409.
- Radcliff KE, Kepler CK, Hellman M, Restrepo C, Jung KA, Vaccaro AR, et al. Does Spinal Alignment Influence Acetabular Orientation: A Study of Spinopelvic Variables and Sagittal Acetabular Version. Orthop Surg. 2014; 6: 15-22.
- Gebhart JJ, Weinberg DS, Bohl MS, Liu RW. Relationship between Pelvic Incidence and Osteoarthritis of the Hip. Bone Joint Res. 2016; 5: 66-72.
- Morimoto T, Kitajima M, Tsukamoto M, Yoshihara T, Sonohata M, Mawatari M. Sagittal Spino-Pelvic Alignment in Rapidly Destructive Coxarthrosis. Eur Spine J. 2018; 27: 475-481.
- Mavcic B, Iglic A, Kralj-Iglic V, Brand RA, Vengust R. Cumulative Hip Contact Stress Predicts Osteoarthritis in DDH. Clin Orthop Relat Res. 2008; 466: 884- 891.
- Mavcic B, Pompe B, Antolic V, Daniel M, Iglic A, Kralj-Iglic V. Mathematical Estimation of Stress Distribution in Normal and Dysplastic Human Hips. J Orthop Res. 2002; 20: 1025-1030.
- Abe H, Sakai T, Ogawa T, Takao M, Nishii T, Nakamura N, et al. Characteristics of Bone Turnover Markers in Rapidly Destructive Coxopathy. J Bone Miner Metab. 2017; 35: 412-418.
- Garnero P, Conrozier T, Christgau S, Mathieu P, Delmas PD, Vignon E. Urinary Type II Collagen C-Telopeptide Levels are Increased in Patients with Rapidly Destructive Hip Osteoarthritis. Ann Rheum Dis. 2003; 62: 939-943.
- Berger CE, Kroner A, Stiegler H, Leitha T, Engel A. Elevated Levels of Serum Type I Collagen C-Telopeptide in Patients with Rapidly Destructive Osteoarthritis of the Hip. Int Orthop. 2005; 29: 1-5.