Editorial
Austin J Otolaryngol. 2014;1(1): 3.
Changes in Gait Performance Caused by Vestibular Lesions
Kazuo Ishikawa*, Yoshiaki Itasaka, Koh Koizumi, Eigo Omi, Kohei Honda and Shinsuke Suzuki
Department of Otorhinolaryngology, Akita Graduate School of Medicine, Head and Neck Surgery, Japan
*Corresponding author: Kazuo Ishikawa, Department of Otorhinolaryngology, Akita Graduate School of Medicine, Head and Neck Surgery, 1-1-1, Hondo, Akita, 010-8543, Japan
Received: May 16, 2014; Accepted: May 20, 2014; Published: May 22, 2014
Editorial
Human gait is a bipedal form of locomotion and is attained through a learning process that occurs after birth. It involves a smooth translation of the body’s center of gravity with minimal energy consumption and is a stable motor behavior with minor interindividual variation. Gait is very important for quality of life. However, gait is affected by many neurological disorders, including disorders of the vestibular system. Accordingly, gait analysis can provide useful information on the path physiological status of patients with vertigo.
Many basic experiments have been undertaken to study the systems involved in the control of gait, and it has been elucidated that a gait initiation signal is transmitted from the mesencephalic locomotor region and cerebellum, especially the fastigial nuclei, to the gait–rhythm releasing system, the muscular tonus control system, and the phase–control system (Figure 1) [1–5]. The phase–control system is divided into ascending and descending pathways, and the descending pathway includes the vestibulospinal system. Thus, it could be thought that peripheral vestibular lesions would mainly affect the phase of gait, and lesions in the dorsal and ventral portion of the mid–pontine tegmentum would mainly affect the pontine muscular control system and result in abnormalities in body weight support during stance in addition to gait rhythm instability.
Figure 1: Subtentorial control system of locomotion.
A unilateral vestibular lesion causes locomotor deviation toward the side of the lesion and decrease of walking speed, and the degree of these alterations correlates with severity of the lesion and indicates the degree of vestibular compensation [6,7]. These gait abnormalities are sometimes referred to as labyrinthine ataxia. However, the effects of unilateral vestibular lesions on gait performance are not restricted to gait deviation.
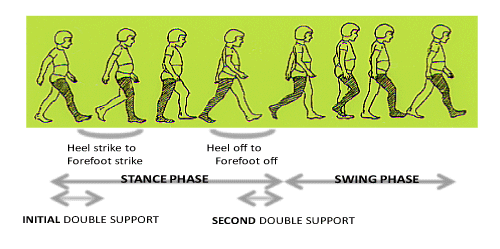
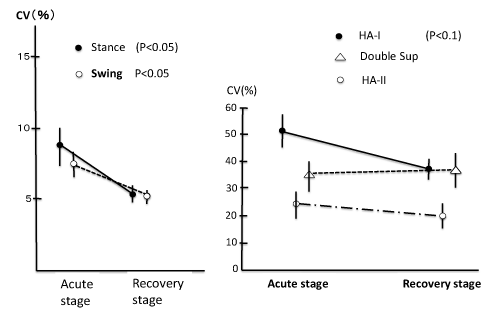
The human gait cycle is composed of stance and swing phases. Stance is largely composed of three sub–phases: Initial double support, single–limb stance, and second double support (Figure 2). The singlelimb stance period corresponds to the swing phase of the other foot. If focusing on foot movement, stance includes the time from heel strike to forefoot strike (HA–I), the single–limb stance period, and the time from heel off to forefoot off (HA–II). The absolute and relative duration of these phases can easily be recorded by the use of foot switches. In normal subjects, the coefficient of variation (CV) of the duration of swing and stance is 6.8–7.0(%) and the coefficient of variation (CV) of HA–I and HA–II is 39.5(%) and 32.2(%) respectively in free gait over a distance of 8 meters [8]. These phase related variables became greater in cases with a vestibular system lesion. The most sensitive variable was coefficient of variation of HA–I (time from heel strike to forefootstrike) in patients with peripheral vestibular system lesion [9]. This might reflect the fact that dorsiflexion of the ankle involves activity of the tibialis anterior muscle, and this muscle has a significantly lower number of neuromuscular units than the gastrocnemius muscle, in addition to a functional connection with the lateral vestibulospinal tract. The instability of stance and swing was greater in patients with a central vestibular lesion than in patients with a peripheral vestibular lesion [10], but the variability of these variables in patients with a peripheral lesion was restored in the recovery stage (Figure 3) [9].
Figure 2: One Gait Cycle.
Figure 3: Changes of gait phase related variables from acute stage to recovery stage in cases with peripheral vestibular lesion [9].
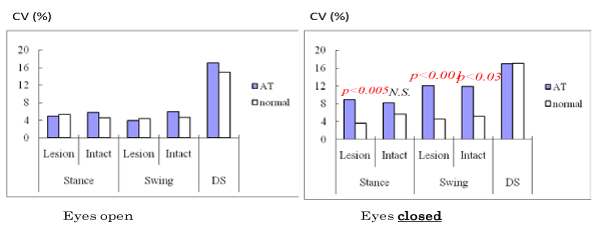
Instability in the timing of gait phases is significantly increased during gait performed with the eyes closed [8]. Feed–forward visual information plays an important role in stable locomotion, and this visual information can compensate for minor functional imbalances in the vestibular system and allow steady locomotion. In fact, patients with small acoustic neuroma had seemingly normal gait with a stable gait pattern when walking with eyes open, but exhibited an increment of CV in the variability of stance and swing phases when walking with eyes closed that was greater than that exhibited by normal control subjects (Figure 4) [11].
Figure 4: CV value changes of gait under eyes open and closed in cases with small acoustic neuroma. Increment of CV value is evident under gait with eyes closed [11].
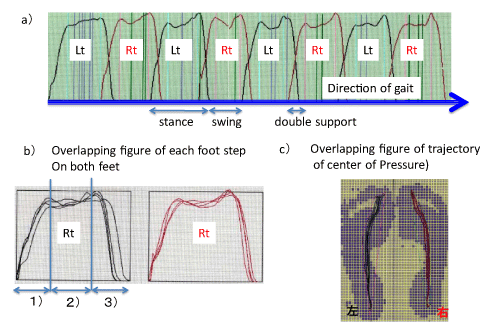
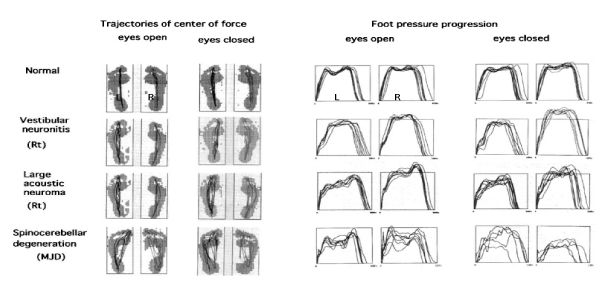
Gait stability can also be quantified by the continual change in vertical foot pressure throughout stance. This can be evaluated by foot pressure progression and the trajectory of the center of pressure during stance that can be recorded by tactile sensors placed under each foot. Representative recordings made using this system are shown in Figure 5. The general gait pattern can be quantified from these recordings and stance, swing, and double support periods can be identified and divided into parts such as body–weight acceptance, body–weight translation and body–weight thrust. Representative recordings from pathological cases (vestibular neuronitis, large acoustic neuroma, and spinocerebellar degeneration) made using this system is shown in Figure 6. When compared with normal subjects, the pathological cases have poor stability of the center–of–pressure trajectories of both feet (Figure 6; left panel) and overlapping figures of foot pressure progression during stance become disordered. (Figure 6; right panel). Spinocerebellar degeneration is the most severely affected of the three pathological cases. The most prominent changes in foot–pressure progression during stance were found in theperiod of body–weight translation, and patients with spinocerebellar degeneration showed the most severe changes [12]. This implies that patients with spinocerebellar degeneration had a discrete vestibular during gait performed with the eyes closed [8]. Feed–forward visual information plays an important role in stable locomotion, and this visual information can compensate for minor functional imbalances in the vestibular system and allow steady locomotion. In fact, patients with small acoustic neuroma had seemingly normal gait with a stable gait pattern when walking with eyes open, but exhibited an increment of CV in the variability of stance and swing phases when walking with system lesion that affected the pontine and cerebellar gait control systems [12,13].
Figure 5: Foot pressure change of gait in normal subject: a) recording of straight walk. b) Overlapping figure of each step; 1): body weight acceptance, 2) body weight translation, 3) body weight thrust . Note the stability.
Figure 6: Representative Recordings of Overlappig Figures of Trajectories of Center of Force and Foot Pressure Progression in three different pathological cases [13].
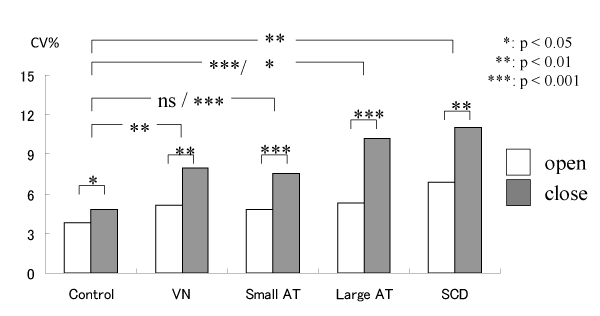
The CV of stance and swing duration also increases in pathological cases, as detailed above, and the results of stance are shown in Figure 7.
Figure 7: Changes of CV (%) of stance in normal and pathological cases: VN: estibular neuronitis, AT: acoustic tumor, SCD: Spinocerebelar degeneration [12].
Gait performance is a whole–body movement, raising the question of what kinds of abnormalities can be detected by threedimensional whole–body motion analyses. Scant data are available to address this question. However, our recent study showed that patients with acoustic neuroma had slower gait speed, shorter stride, and smaller ankle flexion angle at heel strike, and larger pitch and roll head movements than age– and height–matched controls [14] the ankle flexion angle became smaller with larger tumors that were more than 2 cm from the porus acoustics. It is conceivable that head movements in the roll, pitch and yaw planes during locomotion are differentially affected by lesions of superior and inferior vestibular nervous systems, and this should be clarified in future studies.
In addition to gait abnormalities, it has recently been shown that vestibular lesions can affect the navigation system [15]. Furthermore, gait performance can be affected by aging, cognitive function, circulation disorders, malignant tumors, pain, psychological disorders, fear, and neurological disorders, in addition to vestibular system lesions [16,17]. It has recently been shown that regular walking is associated with a lower overall mortality rate [18]. Thus, gait performance has now become a target for study from broad scientific aspects.
References
- Le Ray D, Juvin L, Ryczko D, Dubuc R. Chapter 4--supraspinal control of locomotion: the mesencephalic locomotor region. Prog Brain Res. 2011; 188: 51-70.
- Shik ML, Orlovsky GN. Neurophysiology of locomotor automatism. Physiol Rev. 1976; 56: 465-501.
- Mori S. Integration of posture and locomotion in acute decerebrate cats and in awake, freely moving cats. Prog Neurobiol. 1987; 28: 161-195.
- Mori S, Nakajima K, Mori F, Matsuyama K. Integration of multiple motor segments for the elaboration of locomotion: role of the fastigial nucleus of the cerebellum. Prog Brain Res. 2004; 143: 341-351.
- Mori S, Matsuyama K, Takakusaki K, Kanaya T. The behaviour of lateral vestibular neurons during walk, trot and gallop in acute precollicular decerebrate cats. Prog Brain Res. 1988; 76: 211-220.
- Igarashi M. Squirrel monkey platform runway test. A preliminary report. Acta Otolaryngol. 1974; 77: 284-288.
- Ishikawa K. Apparatus for measurement of dynamic body equilibrium in the guinea pig. Auris Nasus Larynx. 1986; 13 Suppl 2: S35-40.
- Ishikawa K, Edo M, Terada N, Okamoto Y, Togawa K. Gait analysis in patients with vertigo. Eur Arch Otorhinolaryngol. 1993; 250: 229-232.
- Ishikawa K, Edo M, Yokomizo M, Terada N, Okamoto Y, Togawa K. Analysis of gait in patients with peripheral vestibular disorders. ORL J Otorhinolaryngol Relat Spec. 1994; 56: 325-330.
- Ishikawa K, Edo M, Yokomizo M, Miyazaki S, Terada N. Comparative study on gait in patients with acute peripheral vestibular lesion and spinocerebellar degeneration. Equilibrium Res. 1998; 57: 86-89.
- Yin M, Ishikawa K, Omi E, Saito T, Itasaka Y, Angunsuri N. Small vestibular schwannomas can cause gait instability. Gait Posture. 2011; 34: 25-28.
- Angunsri N, Ishikawa K, Yin M, Omi E, Shibata Y, Saito T, et al. Gait instability caused by vestibular disorders - analysis by tactile sensor. Auris Nasus Larynx. 2011; 38: 462-468.
- Ishikawa K, Cao ZW, Wang Y, Wong WH, Tanaka T, Miyazaki S, et al. Dynamic locomotor function in normals and patients with vertigo. Acta Otolaryngol. 2001; 121: 241-244.
- Ishikawa K, Itasaka Y, Saito T, Omi E, Honda K. Three dimensional analysis of gait in patients with vertigo. Barany Society Meeting, Uppsala, Sweden, 2012, (unpublished data)
- Matthews BL, Ryu JH, Bockaneck C. Vestibular contribution to spatial orientation. Evidence of vestibular navigation in an animal model. Acta Otolaryngol Suppl. 1989; 468: 149-154.
- Troosters T, Gosselink R, Decramer M. Six minute walking distance in healthy elderly subjects. Eur Respir J. 1999; 14: 270-274.
- Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004; 292: 1454-1461.
- Hakim AA, Petrovitch H, Burchfiel CM, Ross GW, Rodriguez BL, White LR, et al. Effects of walking on mortality among nonsmoking retired men. N Engl J Med. 1998; 338: 94-99.