Research Article
Austin J Otolaryngol. 2014;1(1): 5.
Changes in Post-treatment Serum BNP Levels in Patients with Obstructive Sleep Apnea Syndrome
Fujioka T*, Iguchi H, Ohya M and Yamane H
Department of Otolaryngology and Head & Neck Surgery, Osaka City University Graduate School of Medicine, Japan
*Corresponding author: Takanori Fujioka, Department of Otolaryngology and Head & Neck Surgery, Osaka City University Graduate School of Medicine,1-4-3, Asahi-machi, Abeno-ku, Osaka 545-8585, Japan
Received: May 18, 2014; Accepted: July 21, 2014; Published: July 26, 2014
Abstract
Objective: Severe sleep apnea syndrome (SAS) is known to cause heart failure. Various treatments are currently available for SAS, and it is important to assess the alleviation of physical burden on the patient as a result of these treatments. We investigated whether SAS treatment improves serum brain natriuretic peptides (BNP) levels.
Subjects and methods: The subject sample comprised 129 patients aged 14–84 years. Apnea hypopnea index (AHI) and serum BNP levels were measured in all patients. Each patient received suitable treatment; 3 months and 6 months after the start of the treatment, AHI and serum BNP level measurements were repeated, and the values for each patient were analyzed.
Results: The correlation coefficients for the associations of baseline AHI and serum BNP levels with those at 3 and 6 months after treatment initiation ranged from 0.6 to 0.8. Treatment-induced variations in AHI and serum BNP levels exhibited similar correlations.
Conclusion: SAS patients receiving treatment showed decreased serum BNP levels regardless of the type of treatment.
Keywords: Brain natriuretic peptides; Sleep apnea syndrome; Treatment; Apnea hypopnea index
Abbreviations
SAS: Sleep Apnea Syndrome; EDS: Excessive Daytime Sleepiness; BNP: Brain Natriuretic Peptide; AHI: Apnea Hypopnea Index; nCPAP: Nasal Continuous Positive Airway Pressure; ANP: Atrial Natriuretic Peptide; NT-proBNP: N-terminal Pro-BNP; RAA: renin– angiotensin–aldosterone
Introduction
Sleep apnea syndrome (SAS) is a combination of nighttime snoring, nocturnal awakening, frequent nocturnal urination, and decreased quality of sleep. SAS leads to excessive daytime sleepiness (EDS), decreased ability to concentrate, and mood changes, which may affect the social activities of patients. SAS is also associated with a high incidence of comorbidities, including cardiovascular diseases, such as hypertension and ischemic heart disease, as well as cerebrovascular disease and respiratory impairment with or without subjective symptoms; these comorbidities may cause sudden death [1].
The concept of SAS was proposed by Guilleminault in 1976 [2]. And it is currently considered a common condition. However, objective studies regarding whether the current treatments are actually suitable for SAS patients have not been conducted. The first and foremost aim of SAS treatment is to prevent complications, among which cardiovascular disease needs particular attention. Patients with SAS, even those without subjective symptoms or clinical problems, increase cardiac load over a prolonged period during sleep, which is believed to result in a condition similar to very mild heart failure. Because of this, among all SAS complications, we focused on heart failure, because it requires long-term treatment and follow-up. Therefore, we examined how the serum brain natriuretic peptide (BNP) levels, a commonly used biomarker in the diagnosis of heart failure in cardiovascular medicine, fluctuate in patients treated for SAS.
Patients and Methods
One hundred twenty nine outpatients aged 14-84 years old who presented with subjective symptoms, including daytime sleepiness, snoring, sleep apnea, nocturnal awakening, and headaches while awake, who were examined by our department for sleep apnea were included in our study. All subjects were strongly suspected of having SAS on the basis of medical history, visual examination of the nasopharynx and larynx, and fiberscopic findings of the larynx, and an apnea hypopnea index (AHI) obtained by sleep study. Sleep apnea was defined as having an AHI of ≥5 with EDS, which is the current generally accepted definition. At the time of the examination, the purpose of our study was explained to the patients, and verbal and written consent was obtained for all data items used. Furthermore, the absence of any pre-existing abnormality was confirmed by plain radiography of the chest and electrocardiography. And in this study, AHI is definited SAS in30 or more.
Serum BNP levels were measured using the chemiluminescent enzyme-linked immunosorbent assay (PATHFAST BNP™, Mitsubishi Chemical Yatron). All patients with serum BNP levels above the normal 18.4 pg/mL as recorded at the baseline examination and during follow-up were asked to consult the department of cardiovascular medicine, where normal cardiac function was confirmed. Moreover, because beta-blockers and diuretics are known to decrease serum BNP levels, we also checked that patients were not taking these medications.
AHI was measured using the apnea monitor (LS-100) manufactured by Fukuda Denshi. After an automated analysis of the results using the analysis software included in the device (Ver.01-03), we visually checked all data and made appropriate corrections. In the present study, all patients had AHI levels suggestive of severe SAS.
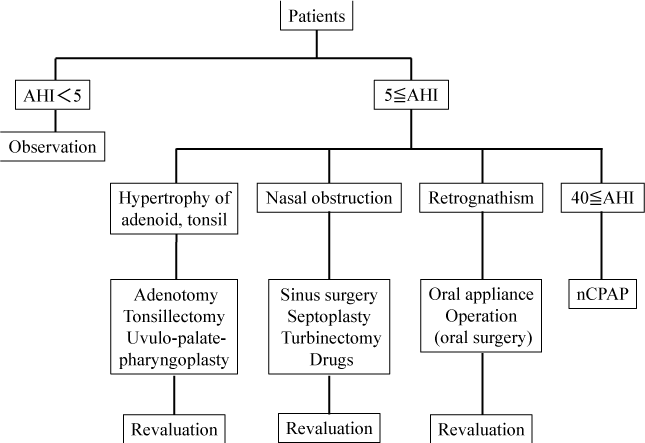
The general choice of treatment method is summarized in Figure 1. AHI was determined on the basis of visual examination of the oropharynx, soft X-ray findings, fiberscopic findings of the larynx, and the apnea monitor. Surgery is usually the treatment of choice when hypertrophy of the palatine tonsils or the adenoids is believed to be the principal cause of SAS. If the symptoms persist after surgery or in those without these findings, nasal continuous positive airway pressure (nCPAP) may be considered. In the present study, however, no patients had pharyngeal abnormalities. NCPAP, which is covered by health insurance in Japan, was chosen by patients with an AHI ≥40 as measured by an apnea monitor. Furthermore, nCPAP was performed using either the REMstar Auto with C-Flex™ or REMstar Auto M Series with C-Flex™. The data obtained from these devices were analyzed using the specialized software “Encore Pro ver.1.8.49.” If the narrowing of the nasal airway was considered the cause of SAS, surgery was performed on the paranasal sinuses, nasal septum, or inferior turbinate surgery was performed, and patients unsuitable for surgery were treated using antiallergic agents (Olopatadine hydrochloride and Cetirizine hydrochloride) or nasal drops (Fluticasone propionate). Furthermore, patients in whom retrognathia-induced pharyngeal narrowing was believed to have caused SAS were requested to wear a mouthpiece. In patients who underwent continuous treatment, AHI and serum BNP levels were measured every 3 months after treatment initiation. For patients using nCPAP, the device was equipped for AHI measurement; therefore, the values obtained from with the device were used.
Figure 1: Outline in selection of medical treatment.
This study was submitted to the Ethical Review Board in our institution.
Results
Study subjects composition
The details of the patients included in our study are shown in Tables 1 and 2. The 47 patients without severe SAS, as indicated by AHI values in 5–30 range, who did not wish to receive treatment, did not undergo subsequent examinations and were instructed to come for regular follow-up. Patients with an AHI of ≥40 were first treated with surgery. Patients who did not wish to use nCPAP underwent nasal treatment or wore a mouthpiece. Patients able to use nCPAP for more than >6 h/day for >4 days/week were included in the favorable prognosis group, and patients using it less were included in the unfavorable prognosis group. Other treatments were recommended when nCPAP use was not possible due to severe discomfort and pain; however, all patients were asked to undergo regular follow-up. Changes in serum BNP levels and AHI were analyzed using a t-test, and correlations between the indices were analyzed.
Treatment
Observation
Nasal drugs
Tonsillectomy
A&T
OA
Sinus surgery
nCPAP
All patients
Number
47
19
13
4
6
1
39
129
Sex
(male)
26
13
11
3
6
1
34
94
(female)
21
6
2
1
0
0
5
35
Age
(year)
47.3±16.1
48.1±17.2
28.5±11.4
15.5±1.3
57.3±16.7
65
52.3±14.9
46.7±17.5
BMI
(kg/m2)
24.58±4.33
24.56±4.90
26.18±7.29
19.40±2.73
24.85±4.11
25.43
29.05±4.96
25.95±5.34
AHI
mean and SD
9.48±5.43
20.31±10.72
38.12±30.40
13.00±9.14
50.98±17.08
40.30±--
54.08±16.00
29.72±24.19
range
0.6-28.0
4.0-41.7
5.4-99.1
4.6-25.8
35.5-75.2
40.3
40.0-80.0
0.6-99.1
median value
8.6
22.4
32.7
11.8
42.3
--
54.2
29.4
Table 1: Clinical characteristics of each medical treatment in this study.
AHI
P value
Treatment
First
Number
3 months
Number
6 months
Number
3 months
6 months
Observation
9.48±5.43
47
*
0
*
0
*
*
Nasal drugs
20.31±10.72
19
14.37±8.39
19
14.95±8.03
19
0.001
<0.001
Tonsillectomy
38.12±30.40
13
7.96±9.21
13
7.58±7.94
13
0.001
0.002
Adenotomy + Tonsillectomy
13.00±9.14
4
2.43±1.99
4
2.15±1.79
4
0.062
0.061
Oral appliance
50.98±17.08
6
19.58±6.14
6
21.58±6.09
6
0.002
0.003
Sinus surgery
40.30±--
1
20.30±--
1
21.50±--
1
*
*
nCPAP
(Favorable prognosis)
60.02±18.18
20
3.05±1.98
20
3.37±1.35
20
<0.001
<0.001
(Unfavorable prognosis + Drop out)
47.84±10.53
19
45.79±11.22
19
47.13±11.11
19
0.078
0.386
serum BNP level (pg/ml)
P value
Treatment
First
Number
3 months
Number
6 months
Number
3 months
6 months
Observation
9.38±3.38
47
*
0
*
0
*
*
Nasal drugs
11.08±4.13
19
9.63±3.66
19
9.72±3.35
19
<0.001
0.012
Tonsillectomy
11.15±3.82
13
7.31±2.44
13
6.58±1.92
13
<0.001
<0.001
Adenotomy + Tonsillectomy
8.30±2.07
4
5.95±0.99
4
5.68±0.71
4
0.063
0.032
Oral appliance
13.22±3.11
6
10.52±3.57
6
8.63±3.41
6
0.003
0.001
Sinus surgery
17.50±--
1
6.20±--
1
6.50±--
1
*
*
nCPAP
(Favorable prognosis)
14.51±3.55
20
7.16±1.84
20
6.48±1.78
20
<0.001
<0.001
(Unfavorable prognosis + Drop out)
14.07±2.88
19
16.15±5.16
19
16.61±7.52
19
0.142
0.198
Table 2: Analysis about transition of an index with the passage of time (paired t-test).
Correlation analysis between indices
Figures 2, 3, and 4 depict a correlation diagram of AHI and serum BNP levels at baseline as well as 3 and 6 months after treatment initiation, as well as the changes in AHI and BNP levels at 3 and 6 months compared with baseline.
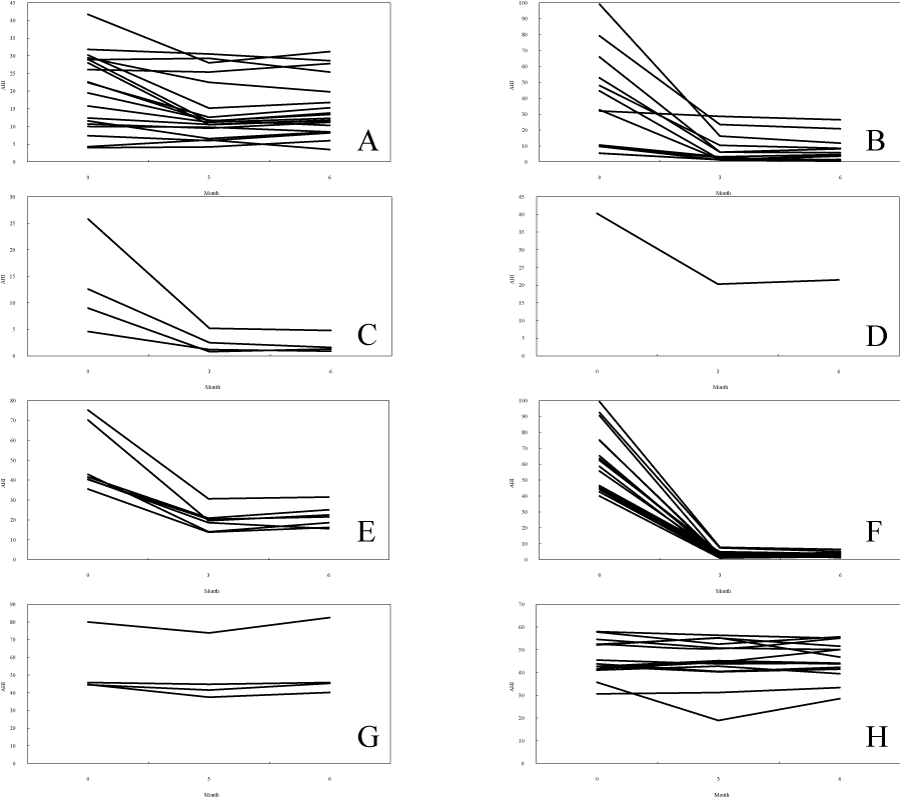
Figure 2: Change of AHI according to the medical treatment. (A) Nasal drugs, (B) Tonsillectomy, (C) Adenotomy + Tonsillectomy, (D) Sinus surgery, (E) Oral appliance, (F) nCPAP user (favorable prognosis), (G) nCPAP user (unfavorable prognosis), (H)nCPAP user (drop out). Each figure showshow AHI changed from the first examination and after 3 months and 6 months after treatment start according to each medical treatment.AHI effectively falls in cases of tonsillectomy, adenotomy+tonsillectomy, sinus surgery, oral appliance, and nCPAP (favorable prognosis).
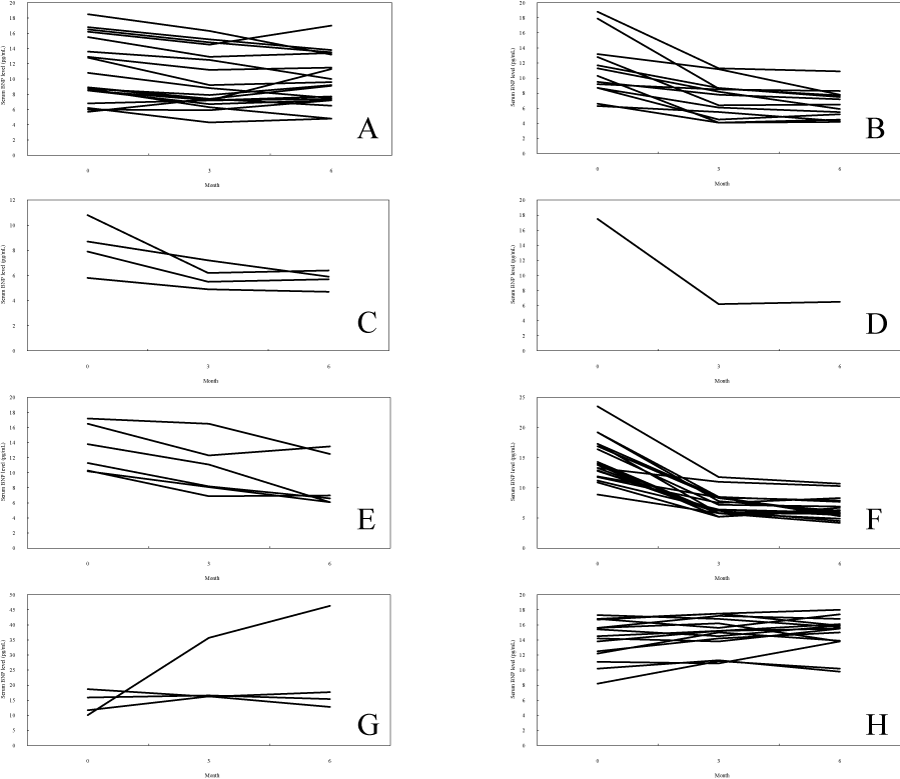
Figure 3: Change of serum BNP level according to the medical treatment. (A) Nasal drugs, (B) Tonsillectomy, (C) Adenotomy + Tonsillectomy, (D) Sinus surgery, (E) Oral appliance, (F) nCPAP user (favorable prognosis), (G) nCPAP user (unfavorable prognosis), (H)nCPAP user (drop out). Each figure showshow serum BNP level changed from the first examination and 3 months and 6 months after treatment start.Serum BNP level effectively falls in cases of tonsillectomy, adenotomy+tonsillectomy, sinus surgery, oral appliance, and nCPAP (favorable prognosis).
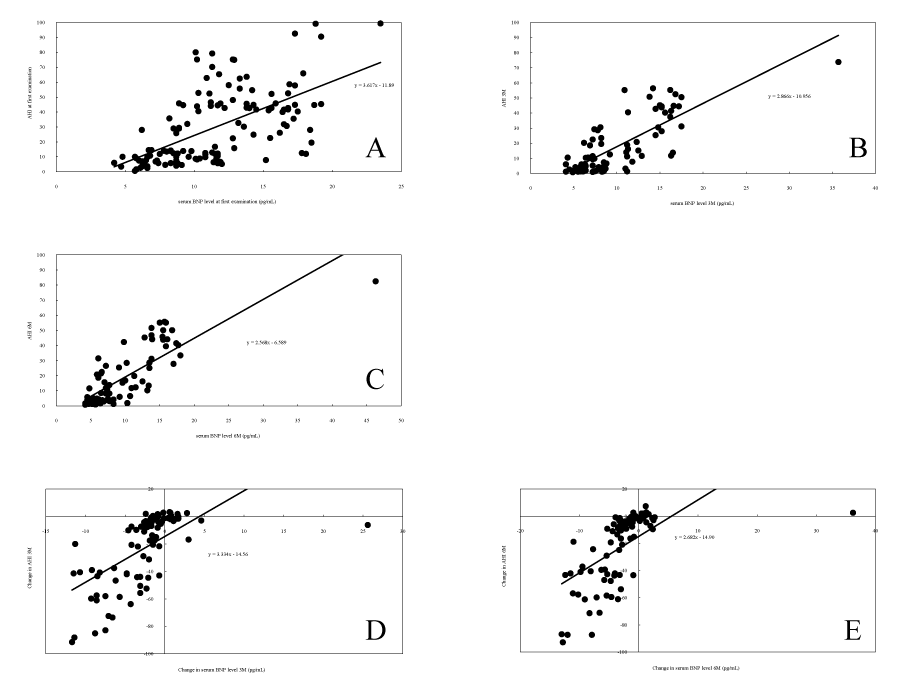
Correlation chart during index.
(A) Correlation between serum BNP level and AHI at the first examination for all patients.
(B) Correlation between serum BNP level and AHI at 3 months after treatment start for all patients.
(C) Correlation between serum BNP level and AHI at 6 months after treatment start for all patients.
(D) Correlation between change in serum BNP level and change in AHI from the first examination to 3 months after treatment start for all patients.
(E) Correlation between change in serum BNP level and change in AHI from the first examination to 6 months after treatment start for all patients.
Each figure shows the correlation between an index and AHI.
Figure 4:Correlation chart during index.
(A) Correlation between serum BNP level and AHI at the first examination for all patients.
(B) Correlation between serum BNP level and AHI at 3 months after treatment start for all patients.
(C) Correlation between serum BNP level and AHI at 6 months after treatment start for all patients.
(D) Correlation between change in serum BNP level and change in AHI from the first examination to 3 months after treatment start for all patients.
(E) Correlation between change in serum BNP level and change in AHI from the first examination to 6 months after treatment start for all patients.
Each figure shows the correlation between an index and AHI.
Spearman’s correlation coefficient was used to calculate the correlation between AHI and serum BNP levels, as shown in Table 3. The values >0.7 were calculated as suggesting strong correlation between AHI and serum BNP levels. The correlation between changes in AHI and in serum BNP levels 3 and 6 months after treatment initiation were 0.777 and 0.811, respectively, indicating a strong correlation between changes in AHI values and those in serum BNP levels. Pearson’s correlation coefficient was also calculated, and a strong correlation of ≥0.7 was observed
1) between index
AHI 0
(P value)
AHI 3
(P value)
AHI 6
(P value)
BNP 0
0.63498
<0.001
BNP 3
0.73017
<0.001
BNP 6
0.77418
<0.001
2) between change in index
AHI 0-3
(P value)
AHI 0-6
(P value)
BNP 0-3
0.77744
<0.001
BNP 0-6
0.81059
<0.001
Table 3: Product-moment correlation coefficient.
Discussion
To date, the treatment for SAS has been chosen on the basis of medical history, presence/absence of upper airway obstruction, imaging findings, serum levels of endocrine parameters, and sleep studies. Although the state of apnea after each treatment has been evaluated, the extent to which the treatments alleviate the cardiac load in each patient has not been evaluated. We then evaluated serum BNP levels at 3 and 6 months after treatment initiation to assess the extent to which SAS treatment eased the cardiac load.
BNP is a hormone that is synthesized and secreted by the heart (predominantly by the ventricles) [3-5]. Myocardial stretching induces an increase in BNP-mRNA expression, and therefore an increase in serum BNP levels [4-6]. BNP is believed to increase Na+ diuresis, promote vasodilation, and inhibit the renin–angiotensin– aldosterone (RAA) system as well as the sympathetic nerve system [7]. In addition, serum BNP levels increase because of hypertension, myocardial infarction, and dilated cardiomyopathy, and heart diseases that causes pressure overload of the ventricles. Renal failure can also increase serum BNP levels. This is attributed to the cardiac load caused by increased body fluid volume as well as decreased renal clearance. In general, the reference value of serum BNP is <18.4 pg/ mL. It has been reported that with an adequate treatment, even in patients with heart disease with increased cardiac load, serum BNP levels may be decreased relatively quickly, while some patients with severe heart failure exhibit serum BNP levels >1000 pg/mL. In addition to BNP, atrial natriuretic peptide (ANP), the new marker N-terminal pro-BNP (NT-proBNP) has been proposed as an index. However, ANP levels fluctuates with each cardiac cycle and daytime exercise, and NT-proBNP is strongly affected by renal function [8,9]. Furthermore, various reports have indicated that ANP and NT-proBNP levels may fluctuate because of SAS or its associated treatments, but there are no definite findings. As a result, we did not use these indices in our study.
The present study shows that serum BNP levels decrease as AHI decreases. In some patients with heart failure, the heart failure was dramatically improved after SAS treatment. This shows that the SAS treatment in itself may be included as part of the treatment for heart failure. We found that in patients with no great cardiac load, serum BNP levels decreased after treatment even when they were within the normal range at baseline. Our study showed that early initiation of SAS treatment eases the cardiac load and may prevent heart failure and cardiovascular disease. It has been theorized that the increase in serum BNP levels due to SAS may be attributed to long-term hypoxia or negative intrathoracic pressure during respiration [8]. Hypoxia triggers the release of reactive oxygen species, the increased secretion of various cytokines, the activation of the sympathetic nerve and RAA systems. In addition, labored breathing all through the night causes intrathoracic negative pressure, increases the preload and after load, and leads to myocardial expansion. The combination of these factors is believed to increase serum BNP levels. If AHI can be decreased by suitable treatment, improved respiration will alleviate the cardiac load, consequently decreasing serum BNP levels. Untreated or inadequately treated SAS, the mortality rate after 7 years can reach ≥30%. Of particular note, compared with healthy individuals, patients with SAS show an approximately 2-fold increase in the incidence of complications (including hypertension), 2–3-fold increase in the incidence of ischemic heart disease, and a 5-fold increase in the incidence of cerebrovascular disease [10]. Although the underlying mechanism is still not fully understood, as mentioned earlier, the onset is considered to be the mild nocturnal increase in cardiac load. In such circumstances pharmacotherapy and lifestyle restrictions are required, which considerably impede the patient’s quality of life and activity of daily living. If an adequate treatment is initiated during this time, good outcomes can be achieved with simple treatments. Therefore, the results of our study suggest that SAS treatment is efficacious and is preventive.
Furthermore, in Japan, exhaustive tests such as the all-night polysomnography are not widely used, and sleep studies are often conducted using simple equipment. Therefore, we hope that the utilization of serum BNP levels will gain popularity in the treatment of SAS as well as that of heart failure.
References
- Rössner S, Lagerstrand L, Persson HE, Sachs C. The sleep apnoea syndrome in obesity: risk of sudden death. J Intern Med. 1991; 230: 135-141.
- Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976; 27: 465-484.
- Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988; 332: 78-81.
- Ogawa Y, Nakao K, Mukoyama M, Hosoda K, Shirakami G, Arai H, et al. Natriuretic peptides as cardiac hormones in normotensive and spontaneously hypertensive rats. The ventricle is a major site of synthesis and secretion of brain natriuretic peptide. Circ Res 1991; 69: 491-500.
- Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. I: Natriuretic peptides. J Hypertens. 1992; 10: 907-912.
- Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994; 90: 195-203.
- Yoshimura M, Yasue H, Morita E, Sakaino N, Jougasaki M, Kurose M, et al. Hemodynamic, renal, and hormonal responses to brain natriuretic peptide infusion in patients with congestive heart failure. Circulation. 1991; 84: 1581-1588.
- Anwaruddin S, Lloyd-Jones DM, Baggish A, Chen A, Krauser D, Tung R. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. J Am Coll Cardiol. 2006; 47: 91-97.
- Hübner RH, El Mokhtari NE, Freitag S, Rausche T, Göder R, Tiroke A, et al. NT-proBNP is not elevated in patients with obstructive sleep apnoea. Respir Med. 2008; 102: 134-142.
- Partinen M, Guilleminault C. Daytime sleepiness and vascular morbidity at seven-year follow-up in obstructive sleep apnea patients. Chest. 1990; 97: 27-32.