Review Article
Austin J Otolaryngol. 2014;1(3): 4.
Velopharyngeal Insufficiency in Children
Abdel-Aziz M*
Department of Otolaryngology, Cairo University, Egypt
*Corresponding author: Mosaad Abdel-Aziz, Department of Otolaryngology, Cairo University, 2 elsalam st., King Faisal, Above El-baraka bank, Giza, Cairo, Egypt
Received: August 21, 2014; Accepted: September 07, 2014; Published: October 10, 2014
Abstract
Velopharyngeal insufficiency (VPI) is the incomplete closure of the velopharyngeal valve during articulation and may be during feeding. The patients presented with hypernasal speech which results in difficult communication with its negative effects on the social life of the family. The problem has many causes that include structural defects either in palatal or in pharyngeal muscles. For better understanding of the underlying cause, good anatomical knowledge should be acquainted. Assessment of patients includes otolaryngologic examination, auditory perceptual assessment, nasometric assessment, and radiologic evaluation. However, flexible nasopharyngoscopy is very important to detect the degree and type of velopharyngeal closure pattern. The condition should be managed through a team approach that includes an otolaryngologist, a speech and language pathologist, an audiologist, a radiologist, an orthodontist, a pediatrician, and a psychologist. Speech therapy can be used for patients with small velopharyngeal gap and in postoperative patients where functional residual VPI is present. Orthodontic treatment with palatal obturator or speech aid prostheses is used for children with VPI who are not surgical candidates for palatal reconstruction, or who have had less than optimal surgical results. Surgical intervention is indicated for patients with structural defects, it is either palatal or pharyngeal procedure that aiming for strengthening and/or narrowing of the velopharyngeal valve.
Keywords: Velopharyngeal insufficiency; Cleft palate; Hypernasality; Speech
Abbreviations
VPI: Velopharyngeal Insufficiency; VPC: Velopharyngeal Closure; CT: Computerized Tomography; MVF: Multiview Video fluoroscopy; MRI: Magnetic Resonance Imaging; APA: Auditory Perceptual Assessment
Introduction
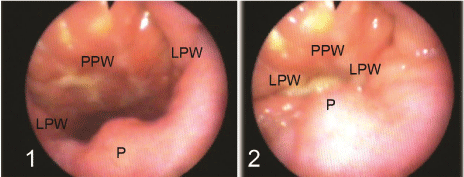
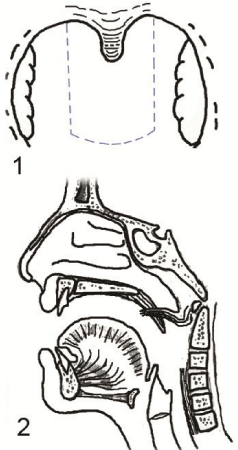
Velopharyngeal valve is the area which is situated between the nasopharynx and oropharynx, it is shuttled during articulation of oral phonemes to prevent escape of words through the nose; a condition which is called velopharyngeal insufficiency (VPI) [1]. This area is bounded by the palate (velum) anteriorly, the posterior pharyngeal wall posteriorly, and the lateral pharyngeal wall on each side (Figure 1). There are 6 muscles controlling the sphincteric mechanism of the velopharyngeal valve, the tensor veli palatini makes the soft palate tense supporting the action of other muscles, the levator veli palatini which is the major elevator of the palate and it forms a sling with the contralateral muscle, the musculus uvulae adds bulk to posterior part of the soft palate making firm contact with the posterior pharyngeal wall, the palatopharyngeus narrows the velopharyngeal valve by adducting the posterior pharyngeal pillars, the palatoglossus pulls the palate anteroinferiorly against the levator sling increasing the velum strengthening, and the superior constrictor produces medial movement of the lateral pharyngeal walls and helps in drawing the palate posteriorly [2].
Figure 1 : Velopharyngeal valve as seen by flexible nasopharyngoscopy 1) open during rest, 2) closed during articulation, P = palate; LPW = lateral pharyngeal wall; PPW = posterior pharyngeal wall.
Etiology
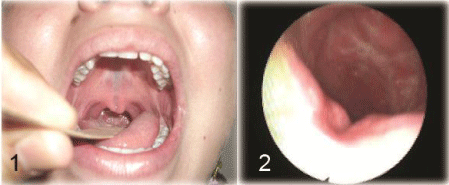
The causes of VPI are variable; however, overt cleft palate even after repair is the most common cause. The incidence of VPI after palatoplasty that may need secondary corrective surgery varies from 15% to 45%, this wide range is due to the presence of different techniques for cleft repair and even every technique is not universally done by different surgeons [3,4]. A submucous cleft palate is another cause for VPI; it is characterized by bifid uvula, muscular diastasis of the soft palate and notching of the posterior border of the hard palate (Figure 2). Anatomically, the levator veli palatini have been shifted from their normal transverse orientation to a longitudinal position with loss of the levator sling. When the uvula is absent without apparent muscular diastasis on oral examination, the condition is termed occult submucous cleft palate which can also cause VPI [2]. Sometimes, VPI may be encountered after adenoidectomy; it is commonly transient due to pain and spasm of palatal muscles, but the condition may be persistent as a large adenoid may compensate for a short or poorly mobile palate, with adenoid removal the mechanism of compensation is eliminated [5]. Also, palatopharyngoplasty which is used for treatment of snoring and sleep apnea, may be complicated with VPI, it is due to either over shortening or excessive fibrosis of the palate [6]. Maxillary advancement used for treatment of maxillary hypoplasia may lead to anterior shift of the palate with incomplete closure of the velopharyngeal valve [7]. Tonsillar hypertrophy is implicated to cause VPI; it causes mechanical impairment of palatal elevation [8]. Hypotonia of palatal muscles can cause VPI as seen in velocardiofacial syndrome, Kabuki syndrome and Down syndrome [2].
Figure 2 : patient with submucous cleft palate 1) oral examination shows bifid uvula and bluish midline of the soft palate, 2) flexible nasopharyngoscopy shows a notched nasal surface of the soft palate.
Clinical Presentation and Evaluation
Patients with VPI are usually presented with hypernasal speech, which is an excessive nasal resonance during vowel production. This hypernasality can render a child’s speech unintelligible and can affect communication. Children with hypernasal speech are often considered less intelligent, less pleasant, and less attractive. Such perceptions may affect the social life of children [1]. Depending on the degree of VPI, the patients may also present with nasal air emissions, weak consonants, short utterance length, obligatory and compensatory articulation errors [9]. During oral examination, the palate should be carefully inspected for abnormal shortening, excessive fibrosis, bifid or absent uvula, and muscular diastasis that appear as a bluish midline of the soft palate (Figure 2). Also, palpation of the posterior border of the hard palated should be performed [10]. Flexible nasopharyngoscopy shows the degree of VPI and the type of velopharyngeal closure (VPC) pattern. There are four VPC patterns, the coronal closure in which the palate is the main contributor in VPC, the sagittal closure in which the lateral pharyngeal walls are the main contributor in VPC, the circular closure in which the palate and lateral pharyngeal walls contribute nearly equal in VPC, and the circular closure with a Passavant’s ridge which is a circular closure with a ridge in the posterior pharyngeal wall. Sometimes, flexible nasopharyngoscopy may show a midline groove on the nasal surface of the soft palate (Figure 2) indicating muscular diastasis [11].
Radiologic evaluation may help in VPI assessment. Plain X-ray in lateral views which taken during rest and during sustained vowel production, it measures the palatal length and elevation as well as the pharyngeal depth. Cineradiography achieves a dynamic assessment of the palate, lateral and posterior pharyngeal wall movements during connected speech. Also, CT scan assesses the mobility of different parts of the velopharyngeal valve with a better illumination. It gives automated numerical values regarding the size of the velopharyngeal gap and the degree of displacement of each wall. However, the effect of gravity in the supine position may alter the palatal mobility, and it needs a co-operative patient and a well experienced radiologist. The Multiview video fluoroscopy (MVF) is the most accepted reliable radiologic tool for the diagnosis of VPI. MVF comprises instillation of barium into the nose; the dye coats the walls of the velopharynx, allowing visualization of all structures in all dimensions during articulation. Its disadvantage is the problem of overlapping that can be overcome by taking multiple views as the technique needs low radiation dosage. While CT gives static shots for the velopharyngeal anatomy, the MVF gives a dynamic imagination with detection of the contributory movement of each wall and the effect of some anatomical features on VPC such as the presence of adenoid pad and/ or the presence of Passavant’s ridge. MRI is a useful investigation that permits different axes with better resolution than MVF and no radiation hazards. However, the use of MRI is limited as a diagnostic tool for VPI in children because it is costly, it needs a cooperative child, and any oral metalwork for dental problems is a restrictive factor [2].
Patients with VPI need speech assessment by a speech and language pathologist. Auditory perceptual assessment (APA) is done by carful listening to the child’s articulation with observation of facies. APA includes commenting on the type and degree of nasality, audible nasal emission of air, consonant precision, compensatory articulatory mechanisms, facial grimace and the overall intelligibility of speech. All these elements are graded along a 5-point scale and the patient is given APA score [1]. Nasometry is unlike APA in its being as an objective method for speech assessment. Nasometer is the most widely used method for acoustic analysis of speech in VPI patients. It measures the nasalance score which is a numeric ratio that reflects the relative amount of the nasal acoustic energy in the patient’s speech. It is a quick non-invasive objective procedure [5,12].
Management
The management of VPI needs an interdisciplinary team that includes an otolaryngologist, a speech and language pathologist, an audiologist, a radiologist, an orthodontist, a pediatrician, and a psychologist. The treatment may be surgical or non-surgical. Speech therapy can be used for patients with small velopharyngeal gap and in postoperative patients where functional residual VPI is present. Patients with anatomical deficiency that prevents adequate closure of the velopharynx cannot be treated with speech therapy. The goal of this therapy is to teach the correct direction of air steam, to teach the correct articulation of all consonants and vowels, and to establish a good coordination of articulatory muscles during VPC. Compensatory articulation errors secondary to VPI is treated by speech therapy as it corrects the functional part rather than the anatomical part of VPI [2,13]. Orthodontic treatment with palatal obturator or speech aid prostheses is used for children with VPI who are not surgical candidates for palatal reconstruction, or who have had less than optimal surgical results. Obturator can substitute tissue deficiency and is attached to the teeth by metal wires, it can be downsized gradually so that the native tissues can strengthen overtime and compensate for the decreasing obturator size [14].
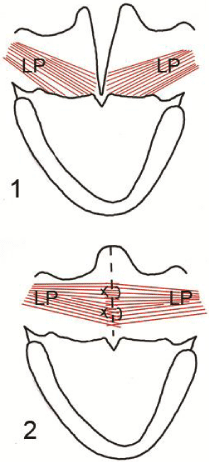
Surgical intervention is indicated for patients with structural defects. Surgical methods can be divided into palatal procedures and pharyngeal procedures. Palatal surgery is designed to improve the palatal muscles function through reorientation of the levator palati muscle, either by Furlow Z-plasty or intravelar palatoplasty techniques (Figure 3). Pharyngeal surgery is dependent on mechanical narrowing of the velopharynx by sphincter pharyngoplasty, pharyngeal flap, or posterior pharyngeal wall augmentation. All these procedures are aimed to improve the VPC and to decrease the gap through which the leakage occurs during speech [2]. Syndromic patients may have medially displaced carotid arteries which raise the risk of hemorrhage during pharyngeal dissection, a problem that can be avoided by carful preoperative imaging [11]. The patients need postoperative speech therapy to improve their articulation abilities. The choice between different surgical methods depends on the data provided by the preoperative evaluation. Two important factors may lead the surgeon to the beneficial method, the status of the levator sling (reconstructed or not) and if reconstructed, what is the type of VPC patterns? [9,15].
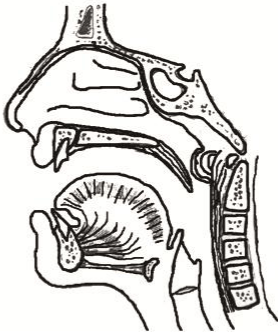
Figure 3 : Illustration shows 1) the abnormal position of the levator palati muscle (LP), 2) after reconstruction of the muscle sling.
Furlow Z-plasty is used to repair cleft soft palate, submucous cleft palate, or as a secondary procedure to lengthen a repaired short palate. Levator palati muscles of both sides are directed horizontally and shifted posteriorly, overlapping each other to reconstruct the levator sling. Also, the lengthening effect of Z-plasty is an important item of the procedure [15]. Intravelar palatoplasty is used as a secondary procedure in post-palatoplasty VPI when the levator sling had not been reconstructed in the primary cleft repair. It includes freeing of the levator palati muscles from their abnormal attachment to the posterior border of the hard palate and from oral and nasal mucosa in the midline and redirection of fibers of both sides medially and closed with mattress sutures [16]. However, the Furlow Z- plasty is superior on the intravelar palatoplasty as there in no palatal lengthening in the latter method which also leaves the lateral fibers in their abnormal place [15].
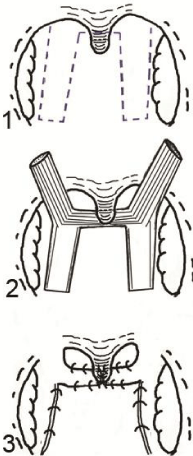
Sphincter pharyngoplasty is used for treatment of VPI with coronal or circular closure pattern. The lateral pharyngeal walls are elevated as a myomucosal flaps that attached together and inserted in a raw area created in the posterior pharyngeal wall at the level of attempted VPC (Figure 4). It preserves the circumferential nature of the velopharyngeal valve with less airway obstructive effect [1,5]. Pharyngeal flap is performed through elevation of the posterior pharyngeal wall to be inserted in the free edge of the soft palate (Figure 5). It acts as a bridge in the middle of the velopharyngeal valve with two lateral gutters for breathing; it is used for patients with sagittal closure pattern as the lateral walls close on the flap during articulation. The procedure has airway obstructive problems [1,2]. Posterior pharyngeal wall augmentation is appropriate for patients with minimal velopharyngeal gap, it can be performed with autogenous tissues or foreign implants (Figure 6). It is rarely used nowadays as its closure effect is less than other pharyngeal procedures [10,17].
Figure 4 : Illustration shows the steps of sphincter pharyngoplasty, 1) Marking of the donor site in the lateral pharyngeal wall; 2) elevation of the myomucosal flaps and creation of a raw area in the posterior pharyngeal wall; 3) suturing of both flaps together and to the posterior pharyngeal wall.
Figure 5 : Illustration shows the steps of pharyngeal flap, 1) marking of the donor site in the posterior pharyngeal wall; 2) elevation of the myomucosal flap and insertion of it into the soft palate.
Figure 6 : Illustration shows posterior pharyngeal wall augmentation.
Conclusion
VPI is not a rare problem in children. It has many causes such as cleft palate even after repair, submucous cleft palate, occult submucous cleft palate, and it may follow adenoidectomy, uveopalatopharyngoplasty, and maxillary advancement procedures. Hypernasal speech with difficult communication is the commonest manifestation. Accurate diagnosis with good understanding of the velopharyngeal closure is essential for treatment. The condition can be treated with speech therapy, orthodontic measures, or surgical procedures followed by speech therapy.
References
- Abdel-Aziz M, El-Hoshy H, Ghandour H. Treatment of velopharyngeal insufficiency after cleft palate repair depending on the velopharyngeal closure pattern. J Craniofac Surg. 2011; 22: 813-817.
- Abdel-Aziz M, Hegazi M, Ghandour H. Velopharyngeal dysfunction. In: Nazario AP, Vermeulen JK, editors. Handbook of Pharyngeal Diseases. Nova Science, New York. 2010; 109–135.
- Bicknell S, McFadden LR, Curran JB. Frequency of pharyngoplasty after primary repair of cleft palate. J Can Dent Assoc. 2002; 68: 688-692.
- Sie KC, Tampakopoulou DA, Sorom J, Gruss JS, Eblen LE. Results with Furlow palatoplasty in management of velopharyngeal insufficiency. Plast Reconstr Surg. 2001; 108: 17-25.
- Abdel-Aziz M, Dewidar H, El-Hoshy H, Aziz AA. Treatment of persistent post-adenoidectomy velopharyngeal insufficiency by sphincter pharyngoplasty. Int J Pediatr Otorhinolaryngol. 2009; 73: 1329-1333.
- Zhao H, Yu R, Niu C. [Complications of PPP: prevention and management strategies]. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 1999; 13: 397-398.
- O'Gara M, Wilson K. The effects of maxillofacial surgery on speech and velopharyngeal function. Clin Plast Surg. 2007; 34: 395-402.
- Abdel-Aziz M. Hypertrophied tonsils impair velopharyngeal function after palatoplasty. Laryngoscope. 2012; 122: 528-532.
- Ysunza A, Pamplona MC, Molina F, Drucker M, Felemovicius J, Ramírez E, et al. Surgery for speech in cleft palate patients. Int J Pediatr Otorhinolaryngol. 2004; 68: 1499-1505.
- Abdel-Aziz M. Treatment of submucous cleft palate by pharyngeal flap as a primary procedure. Int J Pediatr Otorhinolaryngol. 2007; 71: 1093-1097.
- Muntz H, Taylor H, Smith ME. Velopharyngeal dysfunction. In: Cummings CW, Flint PW, Harker LA, editors. Cummings Text book of Otolaryngology Head & Neck Surgery. 4th edn. Philadelphia, PA: Elsevier Mosby. 2005:4086–4098.
- Hirschberg J, Bók S, Juhász M, Trenovszki Z, Votisky P, Hirschberg A, et al. Adaptation of nasometry to Hungarian language and experiences with its clinical application. Int J Pediatr Otorhinolaryngol. 2006; 70: 785-798.
- Golding-Kushner KJ. Therapy techniques for cleft palate speech and related disorders. San Diego CA, Singular publishing. 2001.
- Reisberg DJ. Dental and prosthodontic care for patients with cleft or craniofacial condition. Cleft Palate Craniofac J. 2000; 37: 534–537.
- Abdel-Aziz M, El-Hoshy H, Naguib N, Talaat N. Repair of submucous cleft palate with Furlow palatoplasty. Int J Pediatr Otorhinolaryngol. 2012; 76: 1012-1016.
- Kriens OB. Fundamental anatomic findings for an intravelar veloplasty. Cleft Palate J. 1970; 7: 27-36.
- Sipp JA, Ashland J, Hartnick CJ. Injection pharyngoplasty with calcium hydroxyapatite for treatment of velopalatal insufficiency. Arch Otolaryngol Head Neck Surg. 2008; 134: 268-271.