Research Article
Austin J Otolaryngol. 2014;1(3): 3.
Effect of Amiodarone on Rat’s Isolated Tracheal Smooth Muscle
Hui-Ming Feng1 and Hsing-Won Wang1,2,3*
1Department of Otolaryngology–Head and Neck Surgery, National Defense Medical Center, Republic of China
2Department of Otolaryngology–Head and Neck Surgery, Taipei Medical University, Republic of Chi
3Department of Preventive and Community Medicine, Taipei Medical University, Republic of China
*Corresponding author: Hsing-Won Wang, Department of Otolaryngology–Head and Neck Surgery, Shuang Ho Hospital, Taipei Medical University, No. 291, Jhongjheng Rd., Jhonghe District, New Taipei City, 23561, Taiwan, Republic of China
Received: October 13, 2014; Accepted: October 28, 2014; Published: November 03, 2014
Abstract
Background: Amiodarone is considered the most effective agent to treat supraventricular and ventricular arrhythmia and prevent atrial fibrillation. The blockade of the potassium channel to elongate the refractory period of the myocardium is widely known. Its peculiar pharmacokinetics and toxicity cause some problems to clinicians. The effect on the myocardium, thyroid gland, and lung has been well explored. However, the effect of amiodarone on the tracheal smooth muscle is not well investigated.
Methods: We studied the effectiveness of amiodarone on rat’s isolated tracheal smooth muscle as our preparation. The following assessment of amiodarone were performed: (1) effect on the tracheal smooth muscle resting tension; (2) effect on contraction induced by 10-6 M methacholine as a parasympathetic mimetic; (3) effect on electrically induced tracheal smooth muscle contractions.
Results: The addition of amiodarone at a low dose made no difference to the resting tension of trachea smooth muscle. At 10-4 M of amiodarone, temporary tracheal smooth muscle contraction was detected. When the concentration of amiodarone increased to 3 x 10-4 M, the tracheal smooth muscle relaxed following the brief contraction. For the electrical stimulation and 10-6 M methacholine-induced contraction tests, the amiodarone elicited no scientific change on trachea smooth muscle contraction with increasing concentration.
Conclusion: In the animal model, amiodarone doesn’t change physiologic mechanism of trachea smooth muscle at therapeutic concentration.
Key words: Amiodarone; Trachea; Smooth muscle; In vitro study
Introduction
Amiodarone is a class III action anti-arrhythmic drug to provide QT interval prolonging and myocardial repolarization through voltage gated potassium channel blockade. It also blocks the sodium channel and interferes with β-adrenoceptors and calcium currents [1,2]. The tissues where amiodarone accumulates include the adipose tissue, liver, skeletal muscle, lung, pancreas, thyroid gland, kidney, heart, kidney, skin, adrenal glands, testis, and lymph node [3]. Amiodarone is an iodine-rich drug that may cause thyrotoxicosis in geographical areas with low iodine intake. Besides, this lipophilic agent is a strong inhibitor of lysosomal phospholipase A1 and A2. It can induce direct damage through accumulating in lysosomes, and disturbing metabolic processes, changing the physical properties of cell membranes, inhibiting mitochondrial function, resisting calcium homeostasis, producing reactive oxidant species, and inducing apoptosis [4]. One cause of asthma is contraction of the trachea by parasympathetic dysautonomia [5]. Suppressing the parasympathetic tone can release the asthmatic symptoms. Amiodarone is a complex drug extensively used during resuscitation. The anti-arrhythmic effect of this first aid medicine is well explored, but the effect on the trachea smooth muscle is still unknown. We wanted to research if amiodarone could affect the trachea smooth muscle. The previously documented papers demonstrated one direct and sufficient method to determine the effects of amiodarone on isolated trachea smooth muscle in vitro [6,7]. This device could identify whether amiodarone directly affects the tracheal smooth muscle.
Materials and Methods
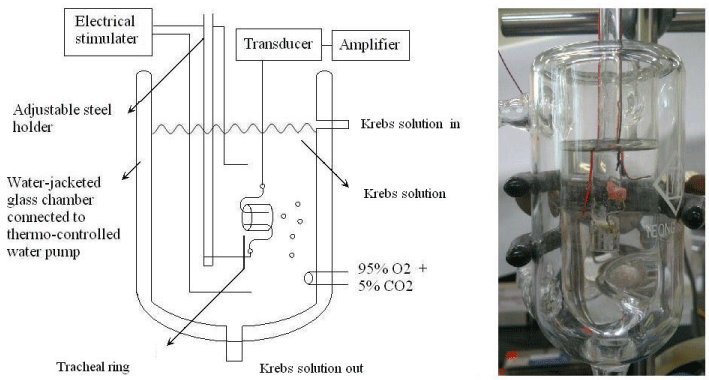
The chemicals used were of the highest purity available. All chemical reagents were obtained from Sigma (St. Louis, MO, USA). We tested methacholine as a tracheal contraction drug. Eighteen adult rats were anesthetized by intraperitoneal administration of pentobarbital (45 mg/kg) and two pieces of trachea about 5 mm in length were removed from each rat. This study was approved by an animal experiment review board (LAC-99-0299). The tracheal specimen was mounted using two steel plates and submersed in a 30 ml muscle bath at 37°C as the previous report (6). Briefly, the bath was filled with 30 ml Krebs solution consisting of (mmol/L) NaCl, 118; KCl, 4.7; CaCl2, 2.5; MgSO4•7H2O, 1.2; KH2PO4, 1.2; NaHCO3, 25.0; and glucose, 10.0. The upper side of the tracheal strip was attached to a Grass FT-03 force displacement transducer (Astro Med, West Warwick, RI, USA) using a steel plate and a 3–0 silk ligature. The other side of the strip was fixed to a steel plate attached to the bath (Figure 1). Passive tension of 0.3 g was applied to the strips and subsequent changes in tension were recorded continuously using Chart v4.2 software (Power Lab, AD Instruments, Colorado Springs, CO, USA). Preliminary tests had shown a tracheal strip immersed in the bath solution used for subsequent experiments did not contract when basal tension was applied. Before drug assays were conducted, isolated tracheas were equilibrated in the bath solution for 15–30 min, during which continuous aeration with a mixture of 95% O2 and 5% CO2 was applied. Stepwise increases in the amount of drugs used were employed to study the contraction or relaxation responses of the tracheal strips. All drugs were administered by adding a defined volume of stock solution to the tissue bath solution. Electrical field stimulation (EFS) (5 Hz, 5 ms pulse duration, at a voltage of 50 V, trains of stimulation for 5 s) was applied to the trachea strip with two wire electrodes placed parallel to the trachea strip and connected to a direct-current stimulator (Grass S44, Quincy, MA, USA). An interval of 2 min was imposed between each stimulation period to allow recovery from the response. Stimulation was applied contiguously to the trachea at 37°C. The following assessments for amiodarone were performed. (1) Effect on tracheal smooth muscle resting tension: this test examined the effect of the drug on the simulating condition of the resting trachea condition. (2) Effect on contraction caused by 10-6 M methacholine (a parasympathetic mimetic): this procedure looked into the postsynaptic events, such as muscle-receptor blockade, enhancement, and second messengers. (3) Effect of amiodarone on electrically induced contractions: electrical stimulation of this tissue causes parasympathetic nerve remnants in the trachea to release transmitter (acetylcholine). If there is interference with transmitter release, electrical stimulation does not cause contraction. Thus, presynaptic events were seen more easily with this procedure. In each experiment, one untreated strip served as a control. The concentrations of the drugs were expressed as concentrations present in the 30 ml bath solution. Data were presented as mean values and standard deviations. Differences between mean values were compared using Student’s t test. Differences were assumed to be significant at P < 0.05.
Figure 1 : Schematic diagram and the actual photo of tension measurements in an isolated rat tracheal strip.
Results
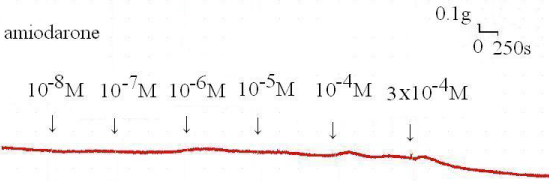
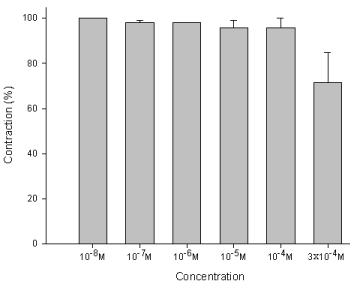
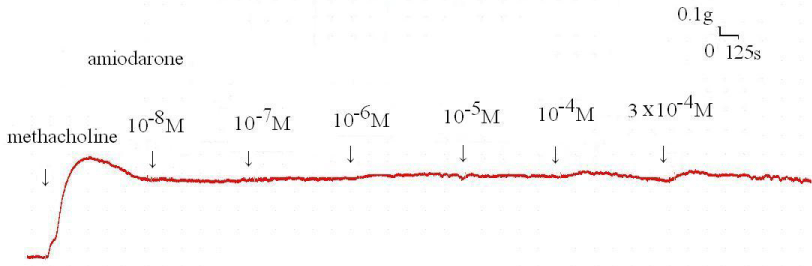
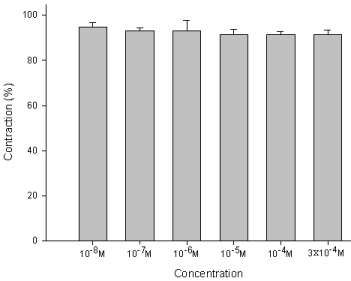
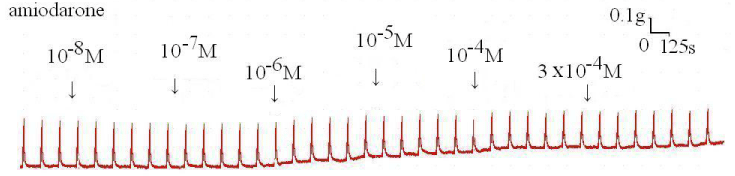
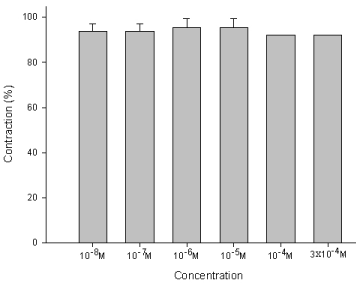
The degree of contraction or relaxation of tracheal strips was estimated from the tension applied to the transducer. Addition of the amiodarone resulted in relaxation of the trachea in resting tension at 3 x 10-4 M (P=0.002<0.05, compared to 10-4 M) (Figures 2,3). Tracheal contraction induced by a small dose of methacholine and electrical stimulation were easily detected. The methacholine-treated trachea displayed a small muscle relaxation after temporary contraction at only 3 x 10-4 M (P=0.019<0.05, compared to 10-8 M). There was no statistical significance could be found in other concentrations (Figures 4,5). No scientific effect of amiodarone at various concentrations on the electrically stimulated trachea was displayed (P=0.35>0.05, compared 3 x 10-4 M to 10-8 M) (Figures 6,7).
Figure 2 : Tension presents in the rat trachea after the application of various amiodarone concentrations. Passive tension was 0.3 g.
Figure 3 : Effect of amiodarone on the basal tension of the trachea: Significant relaxation of the smooth muscle was detected under bathing in high concentrations of amiodarone (3 x 10-4 M). Results were mean + SD (n = 6).
Figure 4 : Original recording of various concentrations of the amiodarone on 10-6 M methacholine-induced contraction of rat’s trachea.
Figure 5 : Effect of amiodarone on methacholine-induced contraction of the trachea smooth muscle: The methacholine-treated trachea displayed muscle relaxation after temporary contraction at only 3 x 10-4 M (P=0.019<0.05, compared to 10-8 M). Results were mean + SD (n = 6).
Figure 6 : Original recording of various concentrations of the amiodarone on electrically induced tracheal smooth muscle contractions.
Figure 7 : Effect of amiodarone on the EFS-induced contraction of trachea smooth muscle: No scientific differences in the contraction of smooth muscles between each concentration of amiodarone baths. Results were mean + SD (n = 6).
Discussion
Amiodarone is a structural analog of the thyroid hormone, and some of its antiarrhythmic actions and its toxicity may be attributable to interaction with nuclear thyroid hormone receptors [6,7]. The therapeutic concentration is around 10-5 M. The tracheal strip we used in this experiment contained intact mucosa, tracheal smooth muscle, cartilage, and connective tissues. Compared to tracheal smooth muscle shredded in vitro at other laboratories, our strip should have more authentically and physically reacted on the extrinsic factors. The methacholine is a commonly used tool in the bronchial provocation test to induce tracheal contraction. The electrical stimulation test is a common experimental model to mimic provocative neuron releasing neurotransmitters. The transducer can detect the extent of smooth muscle contraction to measure the effect of stimulation. In this experiment, the empirical data revealed amiodarone reduced the resting tension of the trachea at a high dose (10-4 M). In addition, the amiodarone has no obvious action on electrical tracheal contraction. The addition of amiodarone with different concentrations to the methacholine-treated tracheal smooth muscle showed significant transient contraction at 3 x 10-4 M. However, the volume of methacholine solution at 3 x 10-4 M, muscle relaxation may occur due to drug toxicity. The amiodarone had no scientifically physiological action on the tracheal smooth muscle within the therapeutic dose range. The different actions of amiodarone on the myocardium and on the trachea in vitro are because of different influences upon the ion channels. The contraction of the tracheal smooth muscle stimulated electrically should be ascribed to vagal excitation. In fact, many modulators of neuronal control are involved in the lower airway, including extrinsic innervations, intrinsic innervations, and reflex regulations [8-11]. Although acetylcholine and the cholinergic agonist’s methacholine are bronchoconstrictors in most species, their potencies vary widely [12]. One of the hypotheses for the different effects of amiodarone on the EFS and MCh test should be diverse stimulation potency. The addition of amiodarone at a high concentration could briefly enhance the influence of methacholine on the trachea smooth muscle. Another possible hypothesis should be the contentiousness effects in amiodarone. Amiodarone can decrease the Ca2+ current, transient outward delayed rectifier, and inward rectifier K+ currents, and exert a noncompetitive adrenergic blocking effect in myocardium. It exhibits acute sympatholytic and vagotonic effects. Its complex reactions may allow for the delicate balance between bronchodilation and bronchoconstriction. The actual interactions between the neuromodulators should be investigated. Therefore, the mechanism of action of amiodarone on the myocardium and trachea may be distinct and requires further study.
References
- Sandhiya S, Dkhar SA. Potassium channels in health, disease & development of channel modulators. Indian J Med Res. 2009; 129: 223-232.
- Morissette G, Ammoury A, Rusu D, Marguery MC, Lodge R, Poubelle PE, et al. Intracellular sequestration of amiodarone: role of vacuolar ATPase and macroautophagic transition of the resulting vacuolar cytopathology. Br J Pharmacol. 2009; 157: 1531-1540.
- Quaglino D, Ha HR, Duner E, Bruttomesso D, Bigler L, Follath F. Effects of metabolites and analogs of amiodarone on alveolar macrophages: structure-activity relationship. Am J Physiol Lung Cell Mol Physiol. 2004; 287: L438-447.
- Baritussio A, Marzini S, Agostini M, Alberti A, Cimenti C, Bruttomesso D, et al. Amiodarone inhibits lung degradation of SP-A and perturbs the distribution of lysosomal enzymes. Am J Physiol Lung Cell Mol Physiol. 2001; 281: L1189-1199.
- Fernandez-Rodriguez S, Broadley KJ, Ford WR, Kidd EJ. Increased muscarinic receptor activity of airway smooth muscle isolated from a mouse model of allergic asthma. Pulm Pharmacol Ther. 2010; 23: 300-307.
- Wang HW, Wu CC. Effects of oxymetazoline on isolated rat's tracheal smooth muscle. Eur Arch Otorhinolaryngol. 2008; 265: 695-698.
- Kao CH, Chu YH, Wang HW. Effects of lidocaine on rat's isolated tracheal smooth muscle. Eur Arch Otorhinolaryngol. 2010; 267: 817-820.
- Ward JK, Barnes PJ, Springall DR, Abelli L, Tadjkarimi S, Yacoub MH. Distribution of human i-NANC bronchodilator and nitric oxide-immunoreactive nerves. Am J Respir Cell Mol Biol. 1995; 13: 175-184.
- Haberberger R, Schemann M, Sann H, Kummer W. Innervation pattern of guinea pig pulmonary vasculature depends on vascular diameter. J Appl Physiol (1985). 1997; 82: 426-434.
- Canning BJ. Reflex regulation of airway smooth muscle tone. J Appl Physiol (1985). 2006; 101: 971-985.
- Wine JJ. Parasympathetic control of airway submucosal glands: central reflexes and the airway intrinsic nervous system. Auton Neurosci. 2007; 133: 35-54.
- Maize DF, Fedan JS, Dey RD. Characterization of neural control and contractile function in airway smooth muscle of the ferret. Pulm Pharmacol Ther. 1998; 11: 57-64.