
Research Article
Austin J Otolaryngol. 2024; 10(1): 1131.
Cochlear Implantation in Inner Ear Malformations
Bencheikh R²; Hjaouj K¹; Oujilal A²; Benbouzid A²; Essakalli L²
¹Resident physician in Otolaryngology, Department of Otolaryngology, Head and Neck Surgery, Ibn Sina University, Hospital, Faculty of Medicine, Mohammed V University, Morocco
²Professor of Otolaryngology, Department of Otolaryngology, Head and Neck Surgery, Ibn Sina University Hospital, Faculty of Medicine, Mohammed V University, Morocco
*Corresponding author: Hjaouj Resident physician in Otolaryngology, Department of Otolaryngology, Head and Neck Surgery, Ibn Sina University, Hospital, Faculty of Medicine, Mohammed V University, Rabat, Morocco. Email: khalil.hjaouj@gmail.com
Received: November 02, 2023 Accepted: December 30, 2023 Published: January 06, 2024
Abstract
Introduction: Cochlear Implantation (CI) has proven to be an effective treatment for severe bilateral Sensorineural Hearing Loss (SNHL). Inner ear malformation is a rare anomaly and occurs in approximately 20% of cases with congenital SNHL. There are particular challenges in the implantation of malformed cochleae, such as in cases of facial nerve anomalies, Cerebrospinal Fluid (CSF) leaks or facial stimulation, and the outcomes may differ depending on the severity of the malformation. The aim of this study was to assess the impact of Inner Ear Malformations (IEMs) on surgical complications and outcomes of cochlear implantation.
Methods: Between 2018 and 2022, 9 patients with inner ear malformations were implanted in our department and completed at least 1 year of follow-up. The age range was between 2 years and 4 months and 5 years (average, 3.36 yr). Auditory performance, receptive and expressive language skills, and production and use of speech were evaluated preoperatively and at least 1 year after implantation.
Results: In the study group, the most common malformation was an isolated Enlarged Vestibular Aqueduct (EVA) (55,5%). Overall, the patients with IEMs showed significant improvement in auditory-verbal skills. In general, the patients who had normal cochleae scored significantly better compared to patients with IEMs.
Conclusion: Based on these findings, cochlear implantation is surgically feasible in patients with common cavity, IP types I and II, and EVA. The surgeon should be ready to make modifications in the surgical approach because of the abnormal course of the facial nerve and be ready to produce special precautions to cerebrospinal fluid gusher. Patients with EVA were the best performers in terms of auditory-verbal skills.
Keywords: Cochlear implant; Congenital hearing loss; Gusher; Inner ear malformation
Introduction
Inner ear malformations are present in about 20% of patients with congenital sensorineural hearing loss [1]. Cochlear implantation in children with congenital deafness is an accepted auditory rehabilitation treatment for more than 20 years [2]. With the advances in radiological imaging over the last decades, new imaging techniques have revealed a wide variety of anomalies of the bony labyrinth. Jackler et al. [1] described the first classification of congenital malformations of the inner ear, which were detected by radiologic imaging. They claimed that the type and severity of IEMs depend on the arrested stage of embryogenesis in a linear developmental model. A further detailed classification based on this theory was made by Sennaroglu and Saatci [3].
In their study, cochlear, vestibular, Semicircular Canal (SCC), Internal Acoustic Canal (IAC), and vestibularcochlear aqueduct malformations were classified into subgroups. As a result of this examination, cochlear malformations were divided into 7 groups (Figure 1) as Michel deformity, common cavity, cochlear aplasia, hypoplasic cochlea, Incomplete Ppartition type I (IP-I), incomplete partition type II (IP-II/Mondini deformity), and incomplete partition type III (IP-III); vestibular malformations were divided into 3 groups as vestibular dilatation, SCC malformations, and IAC anomalies; and vestibular and cochlear aqueduct malformations were divided into 2 groups as vestibular aqueduct anomalies and cochlear aqueduct anomalies.
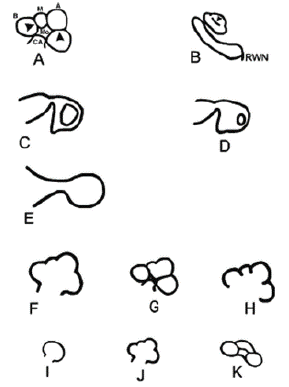
Figure 1: Schematic representation of the normal cochlea and cochlear malformations. (A) Normal cochlea, midmodiolar section. Mo, modiolus; CA, cochlear aperture; B, basal turn; M, middle turn; A, apical turn;arrowheads, interscalar septa. (B) Normal cochlea, inferior section passing through the Round Window Niche (RWN). Arrowhead, interscalar septum between middle and apical turns. (C) Cochlear aplasia with normal vestibule. (D) Cochlear aplasia with enlarged vestibule. (E) Common cavity. (F) Incomplete partition type I. (G) Incomplete partition type II. (H) Incomplete partition type III. (I) Cochlear hypoplasia, bud type (type I). (J) Cochlear hypoplasia, cystic cochlea type (type II). (K) Cochlear hypoplasia, with less than 2 turns (type III) [4].

Figure 2: MRI: Incomplete cochlea and enlargement of the vestibular aqueducts.
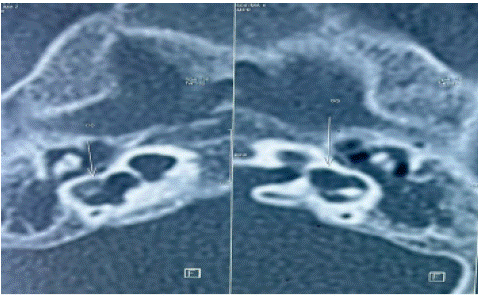
Figure 3: IP-2, with dysplasia of two lateral semicircular canals and bilateral vestibular dilatation.
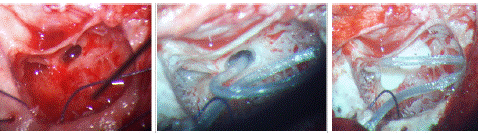
Figure 4: Intraoperative images of IP-II malformation showing CSF oozing.

Figure 5: Inner ear anomalies during embryogenesis according to weeks of gestation.
Cochlear implantation surgery can be performed in all patients with malformation except in those with cochlear aplasia, Michel deformity, and cochlear nerve agenesis. Various complications, such as cerebrospinal fluid leakage, problems in electrode placement, or facial nerve stimulation during activation of the implant, have been reported in CI surgery for patients with inner ear malformations [4-5].
The aim of the study was to present our experience about the intraoperative findings and audiological outcomes of cochlear implants in children with inner ear malformations, and analyze current literature to evaluate outcomes of cochlear implantation in treating SNHL in patients with congenital IEMs, when compared to patients without IEMs.
Methods
This study was performed to analyze intraoperative, postoperative findings, and auditory performance of 9 patients who had inner ear malformations and were treated with cochlear implants at Specialty Hospital–Rabat.
In this retrospective study, 250 patients who underwent cochlear implantation between May 2014 and May 2023 were analyzed at Otorhinolaryngology Department of Specialty Hospital - Rabat.
Nine out of 250 patients who were diagnosed with inner ear malformations were included in the study (Table 1). Computerized Tomography (CT) and MRI scans of these patients were obtained from the local database. Inner ear malformations were diagnosed by radiologists. Patients were classified in accordance with the Sennaroglu and Saatci classification system.
Number
Operation Age
Gender
Radiological Findings
CSF Leak
Facial anormality
1
3yo
F
EVA
Oozing
None
2
3yo
M
EVA
Oozing
None
3
3yo
F
EVA
No findings
None
4
2 yo and 4 months
F
IP – II
Gusher
Procidence
5
5yo
M
IP- I
Gusher
Procidence
6
3yo
F
IP – II
Gusher
None
7
4 yo
M
EVA
No findings
Procidence
8
3yo
M
EVA
No findings
None
9
4yo
M
IP – III
Oozing
None
EVA, Enlarged vestibular aqueduct, IP-I, Incomplete partition type I, IP-II, Incomplete partition type II,
IP-III Incomplete partition type IIII
Table 1: General Information of Patients with Internal Ear Anomalies.
Type of IEM
Radiology
Audiology
Gusher
FN anomaly
Treatment modality
Electrode choice
Complete labyrinthine aplasia
Absent labyrinth
Profound SNHL
Yes
ABI
ABI
Rudimentary Otocyst
Incomplete milimetric otic capsule remnant
Profound SNHL
Yes
ABI
ABI
Cochlear aplasia
Absent Cochlea
Profound SNHL
Yes
ABI
ABI
Common cavity
Round or ovoid cystic structure for cochlea and vestibule
Profound SNHL
Rarey
Yes
CI or ABI
Transmastoid labyrinthotomy or double labyrinthotomy, AVOID MODIOLAR HUDDING ELECTRODE
Cochlear hypoplasia
Cochlear size small, four types+
Conductive, mixed, SNHL
In CH-II Possible
Yes
HA, Stapedotomy, CI or ABI
Thin and short electrode
Incomplete partition-I
Cystic cochlea
Profound SNHL
50% of the cases
Possible
CI or ABI
Electrode with stopper
Incomplete partition-II
Cystic cochlear apex
Normal to profound mixed or SNHL, progressive
Always pulsation but gusher <10% of the cases
Not expected
HA or CI, NO ABI
Any electrode but electrode with stopper preferred
Incomplete partition-III
Modiolus absent, inerscalar septa present
Mixed or SNHL
100% of cases
Yes
HA or CI, NO Stapedotomy, NO ABI
Electrode with stopper AVOID MODIOLAR HUGGING ELECTRODES
Enlarged vestibular aqueduct
Normal cochlea with enlarged VA
Normal to profound mixed or SNHL, progressive
Always pulsation
Not expected
HA or CI, NO ABI
Any electrode but electrode with stopper preferred
Cochlear aperture abnotmalities
Narrow or absent cochlear aperture
Profound SNHL, OAE may be normal, profound SNHL
None
Not expected
CI for CN hypoplasia, or ABI if CN is absent
Standard CI or ABI
IEM: Inner Ear Malformatrion; SNHL: Sensorineural Hearing Loss; FN: Facial Nerve; VA: Vestibular Aquaduct; CN: Cochlear Nerve; HA: Hearing Aid; CI: Cochlear Implantation; ABI: Auditory Branistem Implantation; OAE: Otoacoustic Emissions
Table 2: Various IEMs and their characteristics, operating risks and operating procedures [8].
CI was performed using a retroauricular incision, mastoidectomy, posterior tympanotomy, and oval window or cochleostomy approach under general anesthesia in all patients.
Various brands of cochlear implants were used.
The first implant tune-up was performed 1 month postoperatively. The patients were followed up monthly for 3–4 months and then scheduled for follow-up every 6 months.
All CI patients were evaluated with the Categories of Auditory Perception (CAP) [6] and Speech Intelligibility Rating (SIR) tests [7]. The CAP is an eight-point hierarchical scale that evaluates receptive auditory abilities and ranges from no awareness of environmental sound to telephone use with a familiar talker. The SIR is a five-point hierarchical scale that evaluates expressive abilities and ranges from no intelligible speech to connected speech intelligible to all listeners. The word lists were delivered in the free field at 40 dBSL and the patients were tested in an optimally aided condition with their implants.
Results
Patients’ Characteristics
Amongst the pediatric population that undergone cochlear implantation in our department 9 patients were diagnosed with IEM. The age range at cochlear implantation was between 2 years and 4 months and 5 years (average, 3.36 yr). Of the 9 children, 5 (55.5%) presented with enlarged vestibular aqueduct (LVA), 2 (22.2%) with incomplete partition (IP) Type II (IP II), 1 (11.1%) with IP Type I (IP I) and 1 (11.1%) with IP-III (Table 1).
The preoperative cochlear implantation assessment showed that every patient had severe-to-profound sensorineural hearing loss, without any improvement or residual benefit from well adapted hearing aids.
Regarding the deafness etiology, 4 children (44%) were born of a consanguineous marriage, 1 child (11.11) had suffered from meningitis and 4 children had unknown causes.
Surgical Findings and Postoperative Complications
In all patients, we performed CI surgery via the classical transmastoid-facial recess approach. In 3 patients, the vertical segment of the fascial nerve was located anteromedially toward the promontory, but we did not need to modify the surgical approach. Cochlear implant electrode placement into the scala tympani was performed using the round window technique in 6 (66%) patients and the cochleostomy technique in 3 (33%) patients. The Cochleostomy technique was used in 3 patients because the round window was hidden.
We implanted the Cochlear Nucleus in 6 patients, Oticon in 2 patients, and Advanced Bionics CI system in 1 patient.
CSF gusher was experienced by 3 patients (two with IP-II, one with IP-III, and one with EVA). CSF oozing was encountered in two patients (one with IP III and one with EVA). Cases with CSF gusher required 15–20 min of waiting and ceased completely only after cochlear implant insertion to the cochleostomy site. Full electrode insertion was achieved in all cases with CSF gusher and oozing. The selection of the appropriate electrode has played an important role in achieving this success.
None of the children with CSF gusher or oozing experienced dizziness, excessive vomiting, or CSF leakage in the postoperative period. One patient (IP-II) had meningitis in postoperative period.
Two patients had transient facial paralysis after surgery. One patient had an IP II anomaly (House–Brackmann score of 3) and one patient had EVA (House–Brackmann score of 3). Oral steroids were given to these two patients and tapered in the second postoperative week. All patients fully recovered within six months. The possible etiological factor was neural edema in these two patients. The burr made edema by working close to the nerve due to the narrowness of the window and caused the dissipated heat to reach the nerve.
Speech Perception
Every patient confirmed their cochlear implant daily usage. Post-implantation follow up time ranges from 6 to 45 months (average 25.7 months). After cochlear implantation, the performance of basic auditory skills such as patient response, voice discrimination, and voice recognition increased steadily after cochlear implantation. In the 12th month, basic skill improvement reached 80% in patients with vestibular anomalies and normal inner ear anatomic patients; this improvement was reached later in cochlear anomaly patients. All patients had increased CAP and SIR scores after implantation, those who presentend EVA show better scores than patients with IP-I or IP-II. The average percentage of open set speech recognition was 77% on the monosyllabic test, 100% on the number tests, 66% on the sentences test.
Discussion
Morphologically congenital sensorineural hearing loss can be splitted under two categories. The majority of congenital hearing loss causes (80%) are membranous malformations. Here, the pathology involves inner ear hair cells. There is no flagrant bony abnormality and, thus, in these cases high-resolution computerized tomography and magnetic resonance imaging of the temporal bone reveal normal findings. The remaining 20% have various malformations involving the bony labyrinth and, therefore, can be radiologically demonstrated [8].
This is going through the different weeks that we see these inner ear abnormalities. Basically, they appear during the first trimester between the third and eight week. They progress from complete abnormalities to incomplete abnormalities, and they start from bony abnormalities in the earlier weeks to membranous abnormalities in the later weeks. Also, the abnormalities that occur in the earlier stages of development tend to be more uncommon with about 20% of IEM, as opposed to the IEM that appear later in the development around 7/8 weeks [1].
Recently, owing to advancing technology and imaging methods, inner ear anomalies have been identified as a common cause of congenital hearing loss. For example, inner ear anomaly in the temporal bone has been reported in 20% of patients with congenital hearing loss [1].
Initially, the inner ear malformations were seen as a contraindication to cochlear implantation, but nowadays, this view has completely changed. Cochlear implant surgery can be performed in all patients with inner ear malformation except cochlear aplasia, Michel aplasia, and cochlear nerve agenesis.
3 patients with cochleovestibular anomalies in our study had abnormal facial nerve anatomy (33%). Sennaroglu et al [9] observed abnormal facial nerve anatomy in 4 out of 20 patients in their study (20%). This has been reporte more frequently in patients with inner ear anormalies than in normal patients [10].
Another problem that may be encountered in patients with inner ear malformations is perilymph fistulas. This usually occurs due to defects in the lateral end of the internal auditory canal. If perilymph gusher occurs during surgery, the cochleostomy should be closed completely around the electrode to avoid the risk of meningitis. In our study, gusher was observed in 3 patients with with IP-I and IP-II anomalies. Similar to Sennaroglu et al [9] and Au and Gibsons [11] studies. No gusher was observed in any of the patients with EVA in our study.
Meningitis is a life-threatening complication of cochlear implantation surgery. A study by Biernath et al [12] have shown that the risk of meningitis in patients with CI was 3-fold higher than in the normal population. We report one case of meningitis in our study (11%).
Postoperative hearing and speech rehabilitation programs are of utmost importance for all patients who get cochlear implants. With these programs, patients with cochlear anomalies can also develop their auditory performance at a level observed in patients with normal inner ear anatomy [13,14]. Current studies indicate that early implantation is necessary to gain better communication skills in bilateral total hearing loss. [15] Özdemir et al [16] found that there was a negative correlation between age at the time of operation and development of auditory performance: test scores have increased more rapidly in the small age group. Kim et al [17] have observed that cochlear implantation is beneficial for patients with inner ear malformations and that there was a slight delay in auditory skills in the early post-implantation period compared to normal inner ear anatomic patients. In addition, recent studies also have shown that patients with EVA and patients with normal inner ear anatomy have similar auditory performance [15]. Our study are in line with this, EVA patients show better perception skills.
Although most IEM patients fare well in speech perception 2–3 years after implantation, IEMs such as common cavity deformity or severe cochlear hypoplasia that disrupt cochlear architecture or cochlear nerve formation have limited improvement and tend to perform much worse in all speech perception tests [17,18]. Patients with milder forms of cochlear hypoplasia tend to perform better. Many studies suggest that earlier age of implantation resulted in better performance on open-set word speech perception tests [12].
We did not find severe inner ear malformations like common cavity, which may explain the excellent results presented above concerning speech perception and speech production.
The following conditions should be taken into account to avoid mortal complications such as meningitis: informing the patient’s relatives about all details and risks of the operation, interpreting radiological images carefully, vaccination before the operation, facial monitoring in the course of operation, close follow-up for rhinorrhea in the postoperative period.
Conclusion
There are certain challenges in the management of IEMs: 1. Cerebrospinal fluid gusher and risk for meningitis 2. Facial nerve anomalies 3. Decision making for the surgical approach and the type of electrode. As a result, auditory outcomes of cochlear implantation in patients with inner ear malformations can reach that of CI patients without inner ear anomalies in time. However, it should be known that the facial nerve anomalies and the risk of meningitis are higher in patients with inner ear malformations compared to CI patients without inner ear malformations.
References
- Jackler RK, Luxford WM, House WF. Congenital malformations of the inner ear: a classification based on embryogenesis. Laryngoscope. 1987; 97: 2-14.
- Clark GM, Blamey PJ, Busby PA, Dowell RC, Franz BK, Musgrave GN, et al. A multiple-electrode intracochlear implant for children. Arch Otolaryngol Head Neck Surg. 1987; 113: 825-8.
- Sennaroglu L, Saatci I. A new classification for cochleovestibular malformations. Laryngoscope. 2002; 112: 2230-41.
- Sennaroglu L. Cochlear implantation in inner ear malformations – a review article. Cochlear Implants Int. 2010; 11: 4-41.
- Kim LS, Jeong SW, Huh MJ, Park YD. Cochlear implantation in children with inner ear malformations. Ann Otol Rhinol Laryngol. 2006; 115: 205-14.
- Archbold S, Lutman ME, Marshall DH. Categories of auditory performance. Ann Otol Rhinol Laryngol Suppl. 1995; 166: 312-4.
- Allen MC, Nikolopoulos TP, O’Donoghue GM. Speech intelligibility in children after cochlear implantation. Am J Otol. 1998; 19: 742-6.
- Sennaroglu L, Bajin MD. Classification and current management of inner ear malformations. Balk Med J. 2017; 34: 397-411.
- Sennaroglu L, Sarac S, Ergin T. Surgical results of cochlear implantation in malformed cochlea. Otol Neurotol. 2006; 27: 615-23.
- Buchman CA, Copeland BJ, Yu KK, Brown CJ, Carrasco VN, Pillsbury HC. Cochlear implantation in children with congenital inner ear malformations. Laryngoscope. 2004; 114: 309-16.
- Au G, Gibson W. Cochlear implantation in children with large vestibularaqueduct syndrome. Am J Otol. 1999; 20: 183-6.
- Biernath KR, Reefhuis J, Whitney CG, Mann EA, Costa P, Eichwald J, et al. Bacterial meningitis among children with cochlear implants beyond 24 months after implantation. Pediatrics. 2006; 117: 284-9.
- Rance G, Dowell RC. Speech processor programming. In: Clark GM, Cowan RSC, Dowell RC, editors. Cochlear implantation for infants and children: advances. CA: Singular Publishing Group. 1997; 147-70.
- Barker EJ, Dettman SJ, Dowell RC. Habilitation: infants and young children. In: Clark GM, Cowan RSC, Dowell RC, editors. Cochlear implantation for infants and children: advances. CA: Singular Publishing Group. 1997; 171-90.
- McConkey Robbins AM, Koch DB, Osberger MJ, Zimmerman-Phillips S, Kishon-Rabin L. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Arch Otolaryngol Head Neck Surg. 2004; 130: 570-4.
- Özdemir S, Kiroglu M, Tuncer Ü, Tarkan Ö, Sürmelioglu Ö, Sahin R (Ody). Koklear Implant Uygulanan Hastalarin Isitsel Performans Analizleri. KBB Ihtisas. Dergisi. 2011; 21: 243-50.
- Kim LS, Jeong SW, Huh MJ, Park YD. Cochlear implantation in children with inner ear malformations. Ann Otol Rhinol Laryngol. 2006; 115: 205-14.
- Tucci DL, Telian SA, Zimmerman-Phillips S, Zwolan TA, Kileny PR. Cochlear implantation in patient with cochlear malformations. Arch Otolaryngol Head Neck Surg. 1995; 121: 833-8.