
Case Report
Austin J Otolaryngol. 2024; 10(2): 1136.
Invasive Naso-Sinus and Orbital Mucormycosis
Bencheikh R; Boudinar H*; Benbouzid MA; Oujilal A; Essakalli L
Service d’ORL-CCF, Hôpital des Spécialités, CHU Avicenne Rabat, Morocco
*Corresponding author: Boudinar Houda Service d’ORL-CCF, Hôpital des Spécialités, CHU Avicenne Rabat, Morocco. Email: boudinar1houda@gmail.com
Received: April 22, 2024 Accepted: May 21, 2024 Published: May 28, 2024
Abstract
Mucormycosis is an invasive fungal infection usually observed in immunocompromised patients and especially associated with diabetic ketoacidosis. Mucormycosis is rapidly fatal. The early diagnosis is crucial and is based on imaging data and histology. Amphotericin B must be rapidly initiated and associated with aggressive surgical debridement to reduce mortality.
We report two patients with rhino-orbital mucormycosis and a literature review.
Keywords: Mucormycosis; Diabetes; Amphotericin B; Surgical debridement
Introduction
Mucormycosis is a commonly fatal infection caused by a fungus of the order of Mucorales, with Rhizopus being the most common species associated with this disease [1]. Fungi spores are inhaled causing a sinusitis which eventually spreads to adjacent structures such as the orbit (rhino-orbital mucormycosis) [4]. It is Characterized by a less aggressive and chronical clinical presentation than fulminant mucormycosis that is called indolent mucormycosis [2]. Indolent mucormycosis may also present nonspecific symptoms. It affects the immunocompromised patients and is especially associated with diabetic ketoacidosis [3]. Current data regarding management of indolent mucormycosis is limited due to its rarity. Management of orbital involvement in invasive fungal sinusitis is also controversial due to limited comparative data among treatment strategies [5].
The treatment is based on surgical debridement associated to antifungal drugs as well as the management of diabetic ketoacidosis.
This study presents the clinical findings and treatment of two patients with rhino-orbital mucormycosis.
Case Reports
Case 1
A 46-year-old man with a history of type 1 diabetes consulted at ophthalmology department with a one month history of left eye pain associated with decreased visual acuity. The patient was discharged under local treatment. The evolution was characterized by the installation of chemosis and exophthalmos (Figure 1) associated with diabetic ketoacidosis. The patient was then referred to our department. Nasal endoscopic examination revealed necrotic debris located in the middle third of the left nasal cavity, absence of the left middle turbinate and a septal perforation.
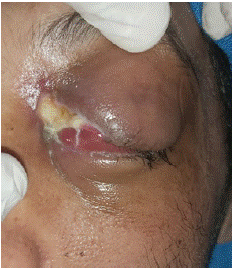
Figure 1: Clinical photography showing chemosis and exophthalmos.
Mycological examination was done on the necrotic tissue of the eyelid and was consistent with a mucormycosis due to Rhizopus.
Standard infection blood work found hyperleukocytosis at 13930 Elements/mm3, as well as an inflammatory syndrome with high rate of sedimentation (90 at the second hour) and elevated CRP (85 mg/l).
Radiological examination by facial CT scan showed a pre-septal cellulitis stage 1 of Chandler’s classification of the left eye with grade 1 exophtalmos.
The patient was put under intravenous amphotericine B, given the lack of clinical nor biological improvement , a control CT scan (Figure 2) and MRI were carried out , which found maxillo-ethmoidal abscesses associated with chandler 5 orbital cellulitis and ipsilateral optic neuritis, thrombosis of the left ICA in its endocranial and cervical portion (Figure 3).
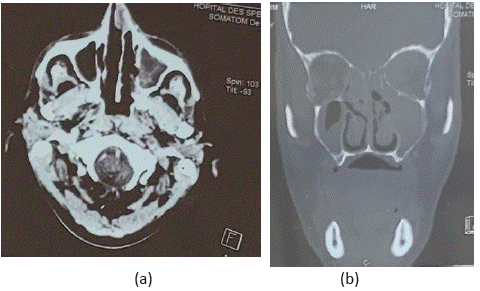
Figure 2: Axial (a) and coronal (b) planes of the orbito-cerebral CT scan showing an invasive left orbital cellulitis with sinus and
basi-cranial extension.
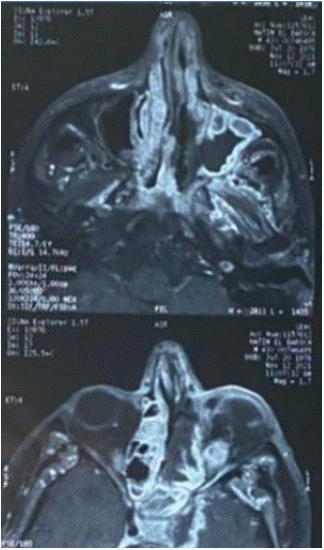
Figure 3: Axial orbito-cerebral revealed maxillo-ethmoidal abscesses.
Thus a surgical treatment was decided which consisted of a middle meatotomy and wide washing of the nasal cavities, then posterior ethmoidectomy, sphenoidectomy and an internal canthal incision was done.
The evolution was marked by clinical (Figure 4), biological, endoscopic and radiological improvement after one year follow-up.
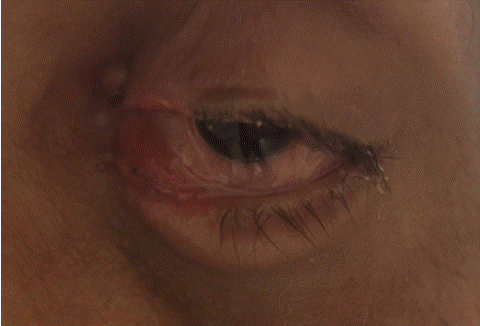
Figure 4: Clinical photography showing chemosis improvement.
Case 2
A 64 years old woman with a history of type 2 diabetes was referred to our department with a history of 1 month left eyelid edema, posterior rhinorrhea and headaches.
Ophthalmic examination found an optic neuropathy.
Nasal endoscopic examination found necrosis of the nasal pyramid and anterior septal perforation.
Radiological examination by facial CT scan (Figure 5) found orbital cellulitis with abscess under the left periosteum stage III of Chandler’s classification. The cerebral MRI confirmed orbital cellulitis and abscess under left periosteum associated with ipsilateral optic neuritis, as well as tight stenosis of the distal segment of carotid artery.
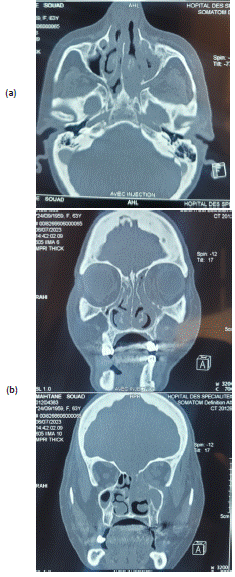
Figure 5: Axial (a) and coronal (b) planes of the orbito-cerebral CT scan showing an invasive left orbital cellulitis with sinusl
extension.
The standard infectious blood work found hyperleukocytosis at 11990 elements/mm3, as well as an inflammatory syndrome with high rate of sedimentation (90 at the second hour) and elevated CRP at 255 mg/l.
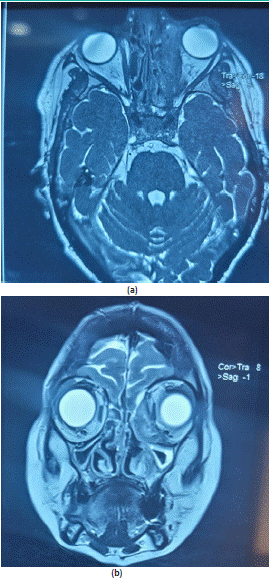
Figure 6: Axial (a) and coronal (b) planes of the orbito-cerebral CT MRI showing a orbital cellulitis and abscess under left periosteum associated with homolaterale optic neuritis.
The patient benefited of surgical treatment that consisted of ethmoidectomy with necrotico-hemorragical lesion removal.
The diagnosis was confirmed by the anatomopathological study that was in favor of mucormycosis. Thus, we associated medical treatment by amphotericine B.
The evolution was marked by clinical, biological, endoscopic and radiological improvement after of two months of follow-up Figure 7.
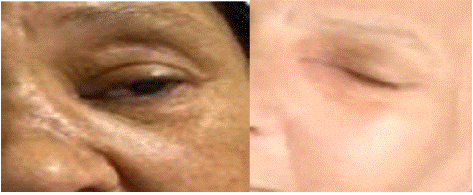
Figure 7: Before (left) and after (right) treatment pictures of second case patient.
Discussion
Mucormycosis was first described by paltauf in 1885 [6]. It is a rare fungal infection, caused by a filamentous fungi belonging to the class of zycomycetes, of Mucorales order, among which 3 germs are most frequently encountered; Rhizopus in 80% of cases (as it is the case of our first patient) followed by Mucor and Absidia [7]; it is currently known that mucormycosis affects mainly immunsuppressed individuals [8], especially uncontrolled diabetic patients with ketoacidosis. Deferoxamine therapy and trauma have also been described as risk factors [9].
The definition of chronic mucormycosis has been controversial, since some authors acknowledge a period of only a few weeks, [10] while others consider the presence of mucormycosis for at least 1 month necessary [2]. Both of our patients had more than one month of infection which is suggestive of mucormycosis. Furthermore, chronic mucormycosis has been associated with internal carotid artery occlusion [10], as was the case for our patient (Figure 8). However, in the study of Cellis Aguilar and al none of the patients developed this complication. The clinical signs are not specific; fever is not always present although prolonged fever is most often encountered [11]. Tissue necrosis with thromboses are manifested by purulent hemo-necrotic rhinorrhea. Patients may also present facial pain and headache. Facial edema and even skin necrosis are also possible [3]. Orbital involvement may consist of eyelid edema, amaurosis, and sometimes even an orbital apex syndrome due to compression [6]. With that said we can as well have strictly asymptomatic patients [11]. Imaging has an important role in the evaluation and management of patients with sinonasal and orbital invasive mucormycosis. CT scan has superior role of characterizing bone erosion, while MRI provides better and earlier visualization of soft tissue damages. The latter has a significant role as soft tissue findings in sinonasal and orbital invasive mucormycosis precedes radiographically significant bone erosion. Thus MRI is the imaging of choice in establishing diagnosis of sinonasal and orbital invasive mucormycosis and evaluating the severity and extent of the disease. And more specifically, MRI may be of utility in distinguishing between devitalized tissue from angioinvasion and surrounding edema [19,20].
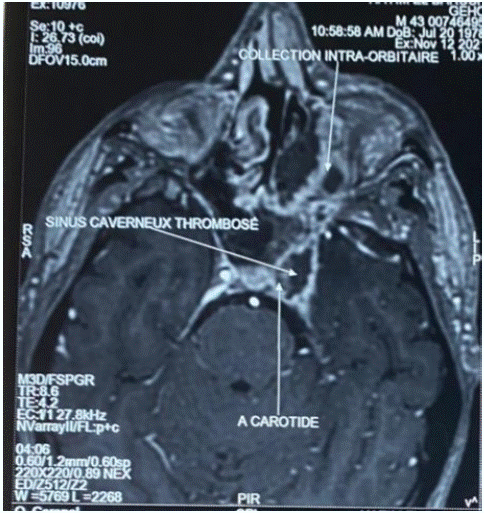
Figure 8: Axial orbito-cerebral MRI showing internal carotid artery occlusion and cavernous sinus thrombosis.
The presence of bone lysis, filling of the sinuses, the extent of the lesions as well as the invasion of the orbit and the base of the skull in computed tomography orient but do not allow to determine the final diagnosis [11]. Magnetic Resonance Imaging (MRI) is indicated to study the extent of the lesions to different structures such as the endocranium, the orbit and the cavernous sinus [11], in our case the MRI revealed orbital involvement and arterial thrombosis. Methods of diagnosis of mucormycosis consists of histopathology, direct testing, and culture of clinical specimens [13].
Invasive sinonasal and orbital mucormycosis is classically managed with systemic antifungal therapy and aggressive surgical debridement; however, the management of indolent mucormycosis is more controversial. Indolent mucormycosis has been successfully managed with systemic antifungal monotherapy [14].
Liposomal amphotericin B is often the antifungal agent of choice due to superior survival rates and its ability to achieve higher blood concentrations without the immediate nephrotoxic side effects commonly seen with the deoxycholate formulation [15]. The treatment can also be managed by surgical debridement alone,[16] or combined medical and surgical therapy [2]. The surgical debridement has a critical role and consists of removing devitalized tissue to allow an adequate concentration of the antifungal treatment to reach the area of active infection; without this crucial step; the prognosis is poor [17,18]. Our first patient was treated initially with intravenous liposomal amphotericin B. Given the lack of improvement, surgical management was deemed necessary. Cornely OA and al recommends extensive and repeated debridement [11].
Conclusion
Naso-sinusal and orbital mucormycosis is a severe and fatal pathology. Its management is based on four factors: (1) early diagnosis, (2) rapid initiation of antifungal therapy, (3) sufficient and aggressive early surgical debridement (4) management of predisposing risk factors, including regulation of glycaemia and adequate management of ketoacidosis.
References
- Ibrahim AS, Kontoyiannis DP. Update on mucormycosis pathogenesis. Curr Opin Infect Dis. 2013; 26: 508–515.
- Celis-Aguilar E, Burgos-Paez A, Villanueva-Ramos N, Solorzano-Barron J, De La Mora-Fernandez A, De Manjarrez-Fernandez A, et al. An emergent entity: indolent mucormycosis of the paranasal sinuses. A multicenter study. Int Arch Otorhinolaryngol. 2019; 23: 92–100.
- Lahmar I, Jerbi S, Chahed H, Fdhila R, Mighri K, Njim L, Para. Nasosinus mucormycosis diagnosis and therapeutic modalities. Tunisian Journal of ENT and Cervico-Facial Surgery. 2008; 21.
- Zhang J, Kim JD, Beaver HA, Takashima M, Lee AG. Rhino-orbital mucormycosis treated successfully with posaconazole without exenteration. Neuro ophtalmol. 2013; 37: 198-203.
- Kalin-Hajdu E, Hirabayashi KE, Vagefi MR, Kersten RC. Invasive fungal sinusitis: treatment of the orbit. Curr Opin Ophthalmol. 2017; 28: 522–533.
- Athanasiadou KI, Athanasiadis DI, Constantinidis J, Anastasiou A, Roilides E, Papakonstantinou E. Successful treatment of rhinoorbital mucormycosis due to Rhizopus arrhizus with liposomal amphotericin B, posaconazole and surgical debridement in a child with neuroblastoma. Medical Mycology. 2019; 25: 10-14.
- Viaud JF, Gin M, Couprie B, Stoll D, Aubertin J. Infections à mucorales chez le patient diabetique. Rev Med Interne. 1994; 15: 541-5.
- Ruoppi P, Dietz A, Nikanne E, Seppa J, Markkanen H, Nuutinen J. Paranasal sinus mucormycosis: a report of two cases. Acta Otolaryngol. 2001; 121: 948–952.
- Wolkow N, Jakobiec FA, Stagner AM, Cunnane ME, Piantadosi AL, Basgoz N, et al. Chronic orbital and calvarial fungal infection with Apophysomyces variabilis in an immunocompetent patient. Surv Ophthalmol. 2017; 62: 70–82.
- Gutierrez-Delgado EM, Trevino-Gonzalez JL, Montemayor-Alatorre A, Cecenas-Falcon LA, Ruiz-Holguin E, Andrade-Vazquez CJ, et al. Chronic rhino-orbito-cerebral mucormycosis: a case report and review of the literature. Ann Med Surg (Lond). 2016; 6: 87–91.
- Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019; 19: e405-21.
- Mimouni O, Curto CL, Danvin JB, Thomassin JM, Dessi P. Sinonasal mucormycosis: case report. European Annals of Otorhinolaryngology, Head and Neck diseases. 2010; 127: 27-29.
- Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and diagnosis of mucormycosis: an update. J Fungi. 2020; 6: 265.
- Mignogna MD, Fortuna G, Leuci S, Adamo D, Ruoppo E, Siano M, et al. Mucormycosis in immunocompetent patients: a case-series of patients with maxillary sinus involvement and a critical review of the literature. Int J Infect Dis. 2011; 15: e533–e540.
- Luna JD, Ponssa XS, Rodrigeuz SD, Luna NC, Juarez CP. Intraconal amphotericin B for the treatment of rhino-orbital mucormycosis. Ophthalmic surg laser. 1996; 27: 706-708.
- Jung H, Park SK. Indolent mucormycosis of the paranasal sinus in immunocompetent patients: are antifungal drugs needed? J Laryngol Otol. 2013; 127: 872–875.
- Wali U, Balkhair A, Al-Mujaini A. Cerebro-rhino orbital mucormycosis: an update. J Infect Public Health. 2012; 5: 116–126.
- Mohamed MS, Abdel-motaleb HY, mobarak FA. Management of rhino-orbital mucormycosis. Saudi Med J. 2015; 36: 865–868
- Gorovoy IR, Kazanjian M, Kersten RC, Kim HJ, Vagefi MR. Fungal rhinosinusitis and imaging modalities. Saudi J Ophthalmol. 2012; 26: 419–426.
- Gorovoy IR, Vagefi MR, Russell MS, Gorovoy M, Bloomer MM, Glastonbury CM. Loss of contrast enhancement of the inferior rectus muscle on magnetic resonance imaging in acute fulminant invasive fungal sinusitis. Clin Exp Ophthalmol. 2014; 42: 885–887.