
Research Article
Austin J Otolaryngol. 2015;2(1): 1023.
Nasopharyngeal Flora of Children Requiring Pressure Equalizing Tubes
Bitar MA1,2,3* and Saade R1,4
1Department of Otolaryngology Head & Neck Surgery, American University of Beirut, Lebanon
2Department of Pediatrics & Adolescent Medicine, American University of Beirut, Lebanon
3Department of ENT Surgery, University of Sydney, Australia
4Dept of Otolaryngology Head & Neck Surgery, MD Anderson, USA
*Corresponding author: Mohamad A Bitar, Department of ENT Surgery, The Children’s Hospital at Westmead, University of Sydney, Cnr Hawkesbury Road and Hainsworth Street, Locked Bag 4001, Westmead NSW 2145 Australia
Received: September 09, 2014; Accepted: January 12, 2015; Published: January 14, 2015
Abstract
Background and Objectives: Children with recurrent middle ear disease (RMED) that is refractory to medical treatment often require ventilation tubes insertion. This may be due to host, environment or microbes related factors. The latter can present a challenge either due to their resistance to antimicrobials or for being uncommon causative pathogens. The aims of this study are to evaluate nasopharyngeal flora in RMED, and to identify associated predictive clinical parameters
Results: We enrolled 84 patients; aged 1 – 9 yrs (mean 3.62). Recurrent respiratory tract infections coincided with RMED in 36.9%. We recovered Hemophilus influenza type b in 23.6%, Moraxella catarrhalis in 14.6%, and Streptococcus pneumoniae in 5.5%. Uncommon RMED bacteria (e.g β Hemolytic streptococcus, Staphylococcus aureus) formed 21.8%; 4.5% of all cultures grew Hemophilus influenza, non-type b. Patients with RMED coinciding with respiratory tract infections, and those with chronic purulent rhinorrhea were more likely to have uncommon RMED organisms (P<0.05).
Conclusion: Children with RMED associated with chronic purulent rhinorrhea, or upper respiratory tract infections may benefit from nasopharyngeal culture to properly guide treatment with antibiotics. This may be an important step before deciding placing tubes in these children.
Keywords: Adenoid; Nasopharynx; Culture; Otitis media; Bacteriology; Nasopharyngeal swab
Introduction
Recurrent middle ear disease (RMED) is a main indication for myringotomy and tympanostomy tube insertion [1]. This may include recurrent acute otitis media (OM) unresponsive to medical treatment, chronic OM with effusion and conductive hearing loss, negative middle ear pressure with impending cholesteatoma, among others. The resistance to medical treatment may be due to host, environment or microbes related factors. The latter can present a challenge either due to their resistance to antimicrobial treatment or for being uncommon causative pathogens.
The classical pathogens of middle ear disease are Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis, in this order. Because of the degree of agreement between their microflora, the nasopharynx has consistently been an intimate suspect of middle ear disease; with the adenoid acting as a reservoir of pathogens [2,3]. Several studies have looked at the nasopharyngeal flora in children with RMED [4], with the classical pathogens of middle ear disease being present in the nasopharynx. However few studies if any specifically addressed the nasopharyngeal flora in the population of children with middle ear disease who failed medical treatment, and required a surgical intervention.
Because these children usually exhaust medical treatment targeted at the usual otitis media pathogens, it would be appropriate to assess the existing nasopharyngeal pathogens and treat them with the appropriate antibiotics before giving up on medical treatment and referring the patients to surgery.
The objectives of this study are to evaluate nasopharyngeal flora in children with RMED requiring ventilation tubes insertion, and to identify associated predictive clinical parameters.
Materials and Methods
After obtaining the IRB (Institutional Review Board) approval and the consent of the caregivers, consecutive children failing medical treatment for RMED and scheduled for ventilation tubes insertion with or without adenoidectomy were prospectively recruited in the study. Recurrence was defined as 4 and more episodes per year, documented by the referring primary care physician. Demographic and clinical data were collected, including age, gender, history of allergy, recurrent OM, recurrent OM with effusion, recurrent upper respiratory tract infections, and obstructing adenoid. The intraoperative findings were recorded; these included tympanic membrane and middle ear fluid’s status, adenoid’s degree of obstruction, friability of the adenoid’s tissue and nature of the nasopharyngeal secretions when present.
Abstract
After tubes insertion, the patient was put in Rose position and the mouth was opened using McIvor mouth gag. A nasogastric tube was inserted through one nasal cavity and pulled out of the mouth to retract the soft palate. The adenoid pad was visualized using a laryngeal mirror. A core biopsy from the adenoid pad was taken using a punch forceps. The specimen was placed in a sterile way into a culture tube and immediately transferred to the lab for processing. The results of the cultures and antibiograms were checked in 3 days and documented. Though it is a more sensitive method, we did not use PCR for the following reasons:
- PCR is not used in routine clinical practice by primarycare physicians.
- PCR can be positive in the absence of live bacteria as it detects their DNA and therefore may not accurately reflect the causative pathogen at time of treatment.
- There was no available budget to use PCR in this study.
The bacteria identified on cultures were classified into 3 groups; group 1 for normal flora, group 2 for the classical OM organisms; and group 3 for other bacteria. The frequencies and means were used to describe the sample, for categorical and continuous variables, respectively. The Kruskal Wallis test was used to compare the different parameters / findings across the 3 groups of bacteria. A p value of 0.05 was considered significant. All analysis was conducted using SPSS software (SPSS Corporation, Chicago, IL).
Results
Eighty-four patients were reviewed, aged 1 – 9 yrs (mean 3.62, SD 1.612), 34 girls and 50 boys. These had a history of allergic rhinitis in 26.2 %, recurrent OM in 53.6%, recurrent upper respiratory tract infections in 48.8% (coinciding with acute OM in 36.9%), persistent effusion in 51.2%, and obstructive adenoid in 76.2%.
A total of 110 organisms were recovered from adenoid cultures. Thirty-seven patients (44%) had one pathogen grown, while 29 patients (34.5%) had 2 pathogens. Sixteen patients (19%) had normal flora, and two patients had sterile cultures (2.5%).
The positive cultures included normal flora in 34.6%, (Group1); Hemophilus influenza type b in 23.6%, Moraxella catarrhalis in 14.6%, and Streptococcus pneumoniae in 5.5%, (Group2) (Table1). Less common acute OM bacteria, (Group 3) (e.g. Beta-hemolytic Streptococcus, and Hemophilus influenza non-type b) formed 21.6% with 4.5% of all cultures growing non-typable Hemophilus influenza (NTHi) (Figure 1). Twenty-one of the 48 regular OM pathogens (43.75%) were resistant to corresponding antibiotics, and 10 of the 24 less common acute OM pathogens (41.67%) were resistant (Figure 2).
Pathogen
N
%
Normal Flora
38
34.55
Hemophilus influenza type b
26
23.84
Moraxella catarrhalis
16
14.55
Streptococcus pneumonia
6
5.45
Hemophilus parainfluenza
6
5.45
Hemophilus influenza non type b
5
4.55
b Hemolytic streptococcus
5
4.55
Staphylococcus aureus
4
3.64
Staphylococcus coagulase negative
1
0.91
Escherichia coli
1
0.91
Enterobacter cloacae
1
0.91
Pseudomonas aeruginosa
1
0.91
Total
110
100
Table 1: Pathogens recovered by tissue culture.
Variable
Kruskal Wallis Test
Df
P
Gender
4.024
2
0.137
Allergic rhinitis
.352
2
0.864
Recurrent OM
.961
2
0.547
Persistent effusion
1.043
2
0.503
Obstructive adenoid
.766
2
0.613
Recurrent URI
3.618
2
0.165
Coinciding OM & URI
7.926
2
0.026
Dull TM
.352
2
0.905
Thick TM
.098
2
0.998
Inflamed TM
3.915
2
0.156
Serous effusion
.687
2
0.665
Mucoid effusion
.698
2
0.740
glue
.619
2
0.717
Purulent effusion
3.945
2
0.159
No effusion
.456
2
0.742
Friable adenoid
.587
2
0.728
Firm adenoid tissue
.587
2
0.728
Clean nasopharynx
4.898
2
0.125
Mucoid nasopharyngeal secretions
1.570
2
0.430
Purulent nasopharyngeal secretions
8.2
2
0.026
Table 2: Predictive ability of various clinical and intraoperative variables towards type of nasopharyngeal flora.
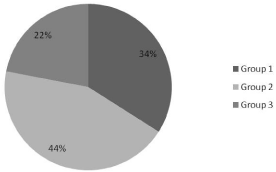
Figure 1: Distribution of the cultured pathogens.
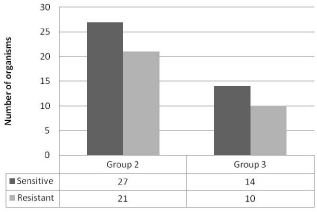
Figure 2: Distribution of the resistant bacteria.
Patients with recurrent OM coinciding with upper respiratory tract infection, and those with purulent nasopharyngeal secretions were more likely to have less common acute OM organisms (P 0.026). No other parameters could predict the nature of the present nasopharyngeal flora (Table 2).
Discussion
Acute OM or OM with effusion is a very common disease in the pediatric age group [5]. Children reaching the surgical option have usually exhausted the medical treatment. The latter often consists of empiric antibiotic course targeting the typical pathogens found in the middle ear fluid and often in the nasopharynx, namely Streptococcus pneumoniae, Hemophilus influenzae, and Moraxella catarrhalis, in this order. Our group of patients revealed a different spectrum of pathogens. The usual OM pathogens formed only 43.7% of the recovered organisms but in the following order of frequency; Hemophilus influenzae, Moraxella catarrhalis, and Streptococcus pneumoniae. This may explain the unsuccessful treatment of some of these patients where high dose amoxicillin was used to target the presumed most common pathogen (i.e. Streptococcus pneumonia), while overlooking the possibility of presence of Hemophilus influenza, Moraxella catarrhalis or others as causative agents.
We did not expect Hemophilus influenza type b to be the most prevalent bacteria in our studied group (23.6%), as all the children were already vaccinated against it. It is also worrisome to start seeing NTHi among the organisms recovered from the nasopharynx of some of the studied children (4.5%). This situation was previously reported in a study by De Carvalho and Kipnis in Brazil, who evaluated the prevalence of encapsulated Hemophilus influenzae and NTHi isolates in the nasopharynx of day-care center children adherent to full vaccination schedule [6]. Hemophilus influenzae carriage rate was 32.1%, while the carriage rate of NTHi was 23.3%, much higher than our group. Looking worldwide, one can see that the incidence of NTHi is increasing. In a study by Gehanno at el., NTHi was found to be the second most common bacteria causing OM after Streptococcus pneumoniae, causing 25–35% of the episodes of acute OM [7].
This change in the bacterial spectrum in OM was also reported by a recent study by Grevers G et al. [8]. Hundred children between the age of 3 months and 5 years with clinically problematic OM had their middle ear fluid cultured through tympanocentesis or sampling of spontaneous otorrhea. The most common recovered pathogen was Hemophilus influenza (where 85.7% were NTHi) followed by Streptococcus pyogenes, Streptococcus pneumonia, and Moraxella catarrhalis.
In another study evaluating the microbiology of OM complicated with otorrhea, Marchisio P et al., looked at 487 positive specimens [9]. The most frequently recovered organism was Haemophilus influenzae (51.0 %), followed by Streptococcus pneumoniae (19.4 %), Streptococcus pyogenes (17.4 %), and Staphylococcus aureus (10.7%).
It is apparent from the above that children with problematic OM (associated with severe signs and symptoms or otorrhea or not responding to empiric antibiotic treatment) is associated with a changing spectrum of middle ear pathogens and nasopharyngeal flora. This may be due to the fact that these children have taken multiple courses of antibiotics targeting one type of organisms and not the other, in addition to being vaccinated against Haemophilus influenza type b and Streptococcus pneumoniae’s vaccine serotypes.
Vaccination alone had its own impact on the nasopharyngeal flora and thus on OM pathogens. The introduction of 7-valent pneumococcal conjugate vaccine in 2000 led to an increase in the relative percentage of acute OM cases caused by NTHi [10]. It also resulted in increase in the occurrence of acute OM caused by nonvaccine Streptococcus pneumoniae serotypes [11]. This increase in the prevalence of NTHi reached 85.7% in clinically problematic otitis media [8]. The impact of pneumococcal vaccine was studied by Casey and Pichichero [12] and Block et al. [10], who compared the bacteriology of middle ear fluids obtained by tympanocentesis before and after the introduction of pneumococcal conjugate vaccine. Both studies showed an increase in the percentage of acute OM caused by NTHi in children with RMED or failing initial empiric antimicrobial treatment. This is consistent with the results of the Finish OM study which reported an increase in OM due to NTHi [11].
Though our incidence of NTHi was not as high as other studies, its emergence in our population needs attention. The importance of this strain of Haemophilus influenza comes from its potential virulence. It has been associated with a history of recurrent OM, treatment failure, concomitant conjunctivitis, bilateral OM, and relapsing OM (i.e. OM recurring within 2 weeks of completing a course of any antibiotic) [13,14].
The virulence of NTHi depends on the geographic location, with 20–35% of NTHi strains, producing beta-lactamase (resistant to amoxicillin). It has been also noticed that NTHi shows another way of resistance by altering the penicillin binding proteins [15].
The present challenge in children with RMED is how to predict the presence of NTHi or other “non-conventional pathogen” as a causative agent. Short of tympanocentesis, which is not a routine practice when assessing every child with OM, nasopharyngeal culture would be a reasonable alternative, as the nasopharynxes play an important role in harboring the pathogens causative of OM [16]. In fact the incidence of OM correlates with the frequency of childhood nasopharyngeal colonization. Children colonized during their first year of life with NTHi, Streptococcus pneumoniae, or Moraxella catarrhalis are at a 4-fold, 4-fold and 2-fold increased risk, respectively, to have recurrent OM when compared with noncolonized children [17,18].
Therefore, we do recommend taking a nasopharyngeal swab for culture in children with recurrent OM or problematic OM to properly direct antibiotic treatment, unless tympanocentesis is available and convenient. Performing PCR testing on the obtained sample will be a plus if readily available and if patient can afford it, as it will fill the gap of the false negative results of routine culture.
We have looked at various clinical parameters trying to see if any one of them can predict the nature of pathogens in the nasopharynx of the studied children. Our findings suggest that only few clinical findings can be of use. These included a history of nasopharyngeal purulence and or the occurrence of OM episodes concomitantly with upper respiratory tract infections. These were found to be associated with recovery of uncommon OM pathogens and thus can be used as an additional clue to perform nasopharyngeal culture to guide a proper antibiotic therapy.
Conclusion
The nasopharyngeal carrier state of children requiring tubes insertion does not reflect the classical OM pathogens. This may have an implication on the medical treatment of children with RMED before they reach surgery, knowing that the microbiology of OM is changing worldwide. It is useful to take a nasopharyngeal swab to guide the antibiotic treatment of children with problematic OM, a history of RMED, or when OM occurs in the context of an upper respiratory tract infection or nasal purulence.
References
- Rosenfeld RM, Culpepper L, Doyle KJ, Grundfast KM, Hoberman A, Kenna MA, et al. Clinical practice guideline: Otitis media with effusion. Otolaryngol Head Neck Surg. 2004; 130: S95-118.
- Hoa M, Tomovic S, Nistico L, Hall-Stoodley L, Stoodley P, Sachdeva L, et al. Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH. Int J Pediatr Otorhinolaryngol. 2009; 73: 1242-1248.
- Dhooge I, Van Damme D, Vaneechoutte M, Claeys G, Verschraegen G, Van Cauwenberge P. Role of nasopharyngeal bacterial flora in the evaluation of recurrent middle ear infections in children. Clin Microbiol Infect. 1999; 5: 530-534.
- Brook I, Gober AE. In vitro bacterial interference in the nasopharynx of otitis media-prone and non-otitis media-prone children. Arch Otolaryngol Head Neck Surg. 2000; 126: 1011-1013.
- Daly KA, Hoffman HJ, Kvaerner KJ, Kvestad E, Casselbrant ML, Homoe P, et al. Epidemiology, natural history, and risk factors: panel report from the Ninth International Research Conference on Otitis Media. Int J Pediatr Otorhinolaryngol. 2010; 74: 231-240.
- de Carvalho CX, Kipnis A, Thörn L, de Andrade JG, Pimenta F, Brandileone MC, et al. Carriage of Haemophilus influenzae among Brazilian children attending day care centers in the era of widespread Hib vaccination.Vaccine. 2011; 29: 1438-1442.
- Gehanno P, Panajotopoulos A, Barry B, Nguyen L, Levy D, Bingen E, et al. Microbiology of otitis media in the Paris, France, area from 1987 to 1997. Pediatr Infect Dis J. 2001; 20: 570-573.
- Grevers G, Wiedemann S, Bohn JC, Blasius RW, Harder T, Kroeniger W, et al. Identification and characterization of the bacterial etiology of clinically problematic acute otitis media after tympanocentesis or spontaneous otorrhea in German children. BMC Infect Dis. 2012; 12: 312.
- Marchisio P, Bianchini S, Baggi E, Fattizzo M, Galeone C, Torretta S, et al. A retrospective evaluation of microbiology of acute otitis media complicated by spontaneous otorrhea in children living in Milan, Italy. Infection. 2013; 41: 629-635.
- Block SL, Hedrick J, Harrison CJ, Tyler R, Smith A, Findlay R, et al. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr Infect Dis J. 2004; 23: 829–833.
- 11. Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001; 344: 403-409.
- Casey JR, Pichichero ME. Changes in frequency and pathogens causing acute otitis media in 1995–2003. Pediatr Infect Dis J. 2004; 23: 824–828.
- Leibovitz E, Asher E, Piglansky L, Givon-Lavi N, Satran R, Raiz S, et al. Is bilateral acute otitis media clinically different than unilateral acute otitis media? Pediatr Infect Dis J. 2007; 26: 589-592.
- McCormick DP, Chandler SM, Chonmaitree T. Laterality of acute otitis media: different clinical and microbiologic characteristics. Pediatr Infect Dis J. 2007; 26: 583-588.
- Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev. 2007; 20: 368-389.
- Faden H, Waz MJ, Bernstein JM, Brodsky L, Stanievich J, Ogra PL, et al. Nasopharyngeal flora in the first three years of life in normal and otitis-prone children. Ann Otol Rhinol Laryngol. 1991; 100: 612-615.
- Harabuchi Y, Faden H, Yamanaka N, Duffy L, Wolf J, Krystofik D. Nasopharyngeal colonization with nontypeable Haemophilus influenzae and recurrent otitis media. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1994; 170: 862-866.
- Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997; 175: 1440-1445.