
Research Article
Austin J Otolaryngol. 2015;2(2): 1027.
Symmetry of VEMP Responses across Swimmers and Non-swimmers
Krishnamurti S*, Brock R and Watts K
Department of Communication Disorders, Auburn University, USA
*Corresponding author: Krishnamurti S, Department of Communication Disorders, Auburn University, 1199 Haley Center, Auburn, AL 36849, USA
Received: October 31,2014; Accepted: January 19, 2015; Published: January 21, 2015
Abstract
The purpose of the current study was to investigate if swimmers show any greater asymmetry (right versus left) related to the nature of their strength and/ or flexibility training on amplitudes of Cervical Vestibular Evoked Myogenic Potentials than non-swimmers. A total of sixteen subjects (eight swimmers and eight non-swimmers) participated in the study. Results of our study showed no significant differences in amplitudes between swimmers and non-swimmers even when corrected for effects of tonic neck muscle activity. Hence the clinical interpretation of these potentials does not appear to be confounded by swimming-related asymmetries.
Keywords: Vestibular Evoked Myogenic Potentials; Swimmers; cVEMP
Introduction
The vestibular end organs (semicircular canals and otolithic organ) play a dominant role in maintaining balance function in humans. Input from the vestibular end organ is often integrated with input from visual and somatosensory systems for postural and equilibrium control by the brain. The semicircular canals help to monitor angular acceleration while the otolithic organs (utricle and saccule) assess linear acceleration. Clinically, semicircular canal function is evaluated by the caloric test via measurement of the vestibular-ocular reflex (VOR). Clinical evaluation of otolithic function is feasible by measurement of Vestibular Evoked Myogenic Potentials (VEMPs).
The VEMPs are short-latency muscle potentials (electromyograms) that are evoked by loud acoustic stimuli [1-4]. There are two types of VEMP responses: 1) cervical VEMPs (cVEMPs) that are recorded from contraction of the sternocleidomastoid muscle (SCM) and 2) ocular VEMPs (oVEMPS) that are recorded from contraction of the extraocular muscles below the eye. VEMPS are mainly composed of two components: 1) a positive peak occurring around 13 ms (P13) followed by a negative peak occurring around 23 ms (N23) following stimulation. The cVEMP has been established as a valid clinical test of saccular and inferior vestibular function [5,6,2-4]. The ipsilateral reflex pathway mediating VEMPs includes the saccule, inferior vestibular nerve, brainstem vestibular nuclei, the descending medial vestibulospinal tract, the accessory nucleus, the accessory nerve, and the motor neurons of the SCM muscle.
Swimmers often use muscles in the arms, legs, trunk, and neck to optimize performance [7]. They may have a preferred side that can cause asymmetries (primary cause) and also develop when strength and flexibility (secondary cause). Also, asymmetries characteristics of technique related to preferred side for breathing are likely to reinforce possible asymmetries among swimmers. The purpose of the current study was to investigate if swimmers who may develop bilateral asymmetries (right versus left) due to strength and/or flexibility training show any greater asymmetry (right versus left) on VEMP amplitudes than non-swimmers.
Methods and Procedure
A total of sixteen subjects (eight swimmers and eight nonswimmers) in the age range of 19 to 30 years participated in the study. The mean age of swimmers was 33 years, 2 months and 23 years, 11 months for non-swimmers. The mean number of years of swimming experience reported by swimmers was 6 years, 4 months, while the mean number of years of swimming experience reported by nonswimmers was less than six months. All the participants had bilateral normal hearing sensitivity as revealed by pure tone audiometry. Participants did not report any history of otologic disorders or any vestibular symptoms. This study received approval for human subject’s research by the Auburn University Institutional Review Board. All subjects signed informed consent prior to participation.
Surface electromyographic (EMG) activity was recorded (Smart EP2, Intelligent hearing system, Miami, USA) with the subject being in a supine position with electrodes on the upper half of bilateral SCM muscles, and a reference electrode on the lateral end of the upper sternum. During the recording, the subject was instructed to keep the head elevated, and turned to the right or left, while in a supine position throughout the entire test. EMG signals were amplified and bandpass filtered between 10 and 1500 Hz, and monitored to maintain muscle activity at a relatively constant level. Two consecutive runs were performed on the same ear to verify the reproducibility, and the results were averaged providing the final response.
Subjects were instructed to lie in the supine position. The surface non-inverting electrodes were placed over the upper half of SCM muscle. The reference electrodes were placed at the sternal notch, and the ground electrode was placed on the forehead. In a supine position without a pillow, the subject rotated his/her head sideways toward one shoulder and lifted the head up 30o on the yaw plane to activate the SCM muscles. To ensure the SCM muscle activation, the subject was instructed to look at a fixed spot on the wall throughout the entire test. The VEMP activities were recorded with a commercial system (Navigator Pro AEP system, Bio-Logic, IL, USA). The VEMP activity was amplified with a gain of 1,000 and were band-pass filtered at 30–3,000 Hz. Tone burst stimuli used to elicit VEMPs (95 dBnHL, rarefaction, 500 Hz, 1-ms rise/fall time and 2-ms plateau) were presented through insert earphones at a repetition rate of 5 Hz. The analysis time was 40 ms and 200 consecutive runs were averaged for each trial. Two consecutive trials were collected for averaging and further analysis.
Response Analysis
The latency p13 is defined as the positive polarity of the biphasic wave that appears at approximately 13 ms, and the latency n23 is defined as the negative polarity of the biphasic wave that appears at approximately 23 ms.
The amplitude is defined as the peak-to-peak p13-n23 maximum energy in μV. VEMP asymmetry ratio (VAR) is defined as the ratio of the inter-aural amplitude difference to the sum of the amplitudes of both ears.
VARs were calculated for the swimmer and non-swimmer groups using the formula below:
Previous studies have indicated that normal individuals have VARs =40% and VARs =40% and =-40% are considered to be within normal limits [5].
Results
All statistical analyses were performed using SPSS 21 (SPSS Software, SPSS, Chicago, IL). P13-N23 amplitude data that were calculated as shown above were first subjected to a Kolmogorov- Smirnov test of normality. A p value of 0.20 was obtained, rejecting the alternative hypothesis and showing that the data were normally distributed.
In the current study, all of the sixteen subjects showed VEMPs using a 95 dB HL tone burst stimulation but there is a considerable scatter in VARs across subjects for non-normalized VEMP responses (Figure 1) obtained when effects of tonic EMG activity were not accounted for in analyses. Comparing the right–left difference of the relative amplitudes revealed that eleven subjects had larger amplitude on the right side, and five subjects had larger amplitude on the left. Of the non-normalized VARs across sixteen subjects, there were three (of eight) asymmetries noted for the swimmers and three (of eight) asymmetries also seen for non-swimmers. Figure 2 also shows considerable scatter for VARs for normalized VEMP amplitude data that were obtained by dividing the VEMP amplitude by the tonic EMG amplitude for each subject. After correcting for tonic EMG activity across ears, four (of eight) non-swimmers showed significant (>40%) asymmetry on VARs while three (of eight) swimmers showed significant asymmetries on VARs. Repeated measures analyses of variance performed on left ear versus right ear P13-N23 VEMP amplitude data showed no significant differences between interaural VEMP amplitude differences across left and right ears (F{1, 14}=3.99; p>0.05). There were no significant differences between interaural VEMP amplitude differences across swimmers and non-swimmers (F{1, 14}= 4.07; p>0.05).
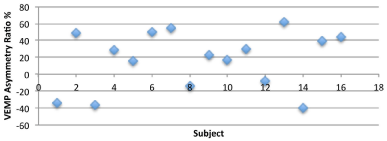
Figure 1: Amplitude Asymmetry ratio across right and left ears for nonnormalized
VEMP responses.
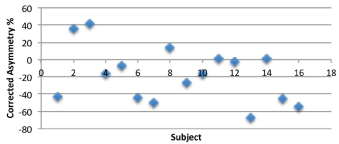
Figure 2: Amplitude asymmetry ratio across right and left ears for normalized VEMP responses.
Because the VEMP amplitude is a parameter used to interpret the response clinically, the influence of tonic EMG level on the VEMP amplitude is must be controlled for the accurate interpretation of interaural VEMP amplitude differences. For this control, we computed ratio data by dividing the peak-to-peak c-VEMP amplitude by the mean rectified EMG level based on recommendations from previous studies [8]. Repeated measures analyses of variance showed no significant differences across left and right ears (F{1, 14}=4.03; p>0.05). There were no significant differences between interaural VEMP amplitude differences across swimmers and non-swimmers (F{1, 14}= 1.88; p>0.05). It is important to note however, that the lack of significant differences between swimmers and non-swimmers may be related to variability in the VEMP amplitude data (see means and standard deviations shown in Figure 3).
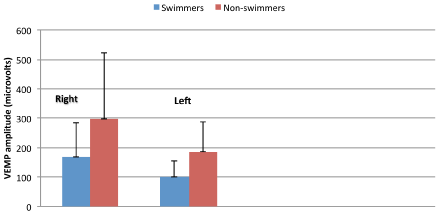
Figure 3: Means and standard deviations for VEMP amplitudes.
Discussion
The clinical interpretation of the VEMP has focused primarily on the amplitude asymmetries or hearing sensitivity differences across right and left ears. Typically, an asymmetry ratio greater than 40% on VEMP testing is considered significant, but this can be affected by the patient’s inability to generate large and symmetrical tonic EMG activity from both the right and left SCMs [8]. Hence uncorrected asymmetry values from VEMPs may lead to spurious findings if the tonic EMG activities across SCMS are not taken into account. One way to normalize the amplitudes is by dividing the VEMP amplitude by the tonic EMG amplitude for each subject. This leads to a corrected VEMP value that may be more suitable for accurate clinical interpretations of VEMP amplitude asymmetries. Because swimmers often show asymmetries related to preferred side for breathing or differences in strength/flexibility, we hypothesized that asymmetry in tonic activity in neck muscles would be measurable in the form of greater asymmetries in swimmers (re: non-swimmers). Hence, while swimmers may develop bilateral asymmetries (right versus left) due to swimming technique and differences in strength or flexibility, those asymmetries do not appear to induce greater asymmetry on VEMP than non-swimmers.
References
- Colebatch JG, Halmagyi GM. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992; 42: 1635-1636.
- Didier A, Cazals Y. Acoustic responses recorded from the saccular bundle on the eighth nerve of the guinea pig. Hear Res. 1989; 37: 123-127.
- Murofushi T, Curthoys IS, Topple AN, Colebatch JG, Halmagyi GM. Responses of guinea pig primary vestibular neurons to clicks. Exp Brain Res. 1995; 103: 174-178.
- Uchino Y, Sato H, Sasaki M, Imagawa M, Ikegami H, Isu N, et al. Sacculocollic reflex arcs in cats. J Neurophysiol. 1997; 77: 3003-3012.
- Akin FW, Murnane OD. Vestibular evoked myogenic potentials. In: Balance function assessment and management. Jacobson G, Shepard N, Editors. Plur Pub. 2008.
- Colebatch JG, Halmagyi GM. Vestibular evoked potentials in human neck muscles before and after unilateral vestibular deafferentation. Neurology. 1992; 42: 1635-1636.
- Sanders RH, Thow J, Fairweather MM. Asymmetries in swimming: where do they come from? J Swimming Res. 2011; 18: 1-9.
- Akin FW, Murnane OD, Panus PC, Caruthers SK, Wilkinson AE, Proffitt TM. The influence of voluntary tonic EMG level on the vestibular-evoked myogenic potential. J Rehabil Res Dev. 2004; 41: 473-480.