
Case Report
Austin J Otolaryngol. 2015; 2(8): 1059.
Metastatic Renal Cell Carcinoma Manifesting Solely as a Vascular External Ear Mass
Rangachari V*
Department of Otorhinolaryngology, Max Super Speciality Hospital, India
*Corresponding author: Vijay Rangachari, Department of Otorhinolaryngology, Max Super Speciality Hospital, Malsi, Mussoorie Diversion Road, Dehradun, PIN 248001, India
Received: July 31, 2015; Accepted: September 30, 2015; Published: October 02, 2015
Abstract
This is a rare case report of a 57 year old lady who came to our hospital with left ear fullness and tinnitus of three months duration. Clinical examination showed a soft, reddish, compressible mass filling the left ear canal that was bleeding to touch. Contrast Computed Tomography (CT) Scan of temporal bones revealed a mass in the left external auditory canal eroding the floor of the canal extending into the mastoid antrum and abutting the descending facial nerve canal. The patient underwent an angiography and feeders from the mastoid and posterior auricular branch of the left occipital artery were embolised. Subsequently, mastoid exploration was done and the lesion was excised. Histopathology was reported as a case of metastatic renal cell carcinoma. Unprecedented manifestation of head and neck metastasis of renal cell carcinoma is discussed along with a relevant literature review.
Keywords: External ear mass; Renal cell carcinoma; Metastasis
Abbreviations
RCC: Renal Cell Carcinoma; DSA: Digital Subtraction Angiography; CT: Computed Tomography; CD 10: Cluster of Differentiation 10; CK: Cyto Keratin; CEA: Carcino Embryonic Antigen; PAS: Para Amino Salicylic Acid; PET: Positron Emission Tomography; FDG: Fluro Deoxy Glucose; cGy: Centi Gray; S3: Third Sacral vertebra; L2: 2nd Lumbar vertebra
Introduction
Renal Cell Carcinoma (RCC) is a lethal tumour that accounts for approximately 3% of all adult malignancies and associated with approximately 13000 deaths annually [1]. Conventional symptoms like hematuria, pain in the flank and a lump in the abdomen are no longer the initial features of presentation of RCC. Advances in radio diagnosis have now resulted in most of these lesions being seen as incidental findings on imaging done for other illnesses. Therefore, there is a predilection for earlier diagnosis of RCC in the asymptomatic stage itself. As a consequence, there is a shift in the staging of the cancer and diagnosis at the time of locally confined renal disease has become frequently possible. Early diagnosis and quick administration of multiple treatment modalities has resulted in changing the outcomes towards a more favorable course.
Case Presentation
A 57 year old lady presented to us with left ear fullness and tinnitus of three months duration. Clinical examination showed a soft, reddish, compressible mass filling the left ear canal whose lower border was not visible. The lesion was bleeding on touch. The normal tympanic membrane could be seen behind the swelling after gentle manipulation. Contrast CT Scan of temporal bones revealed a mass in the left external auditory canal eroding the floor of the canal extending into the mastoid antrum and abutting the descending facial nerve canal (Figure 1). Pure tone audiogram showed a minimal conductive hearing loss in left ear. In view of the vascular nature of the lesion, it was decided to do a Digital subtraction angiography (DSA) to identify the feeder vessels and embolize them. The patient underwent an angiography and feeders were identified from the mastoid and posterior auricular branch of the left occipital artery (Figure 2a). The feeders were embolized using gel foam particles. Post embolization, there was an eighty percent reduction in the tumour blush (Figure 2b). 48 hours post-embolization, she underwent mastoid exploration and the lesion was excised from the external auditory canal and mastoid regions. The facial nerve canal was seen intact and the middle ear was normal. Post-operative recovery was uneventful.
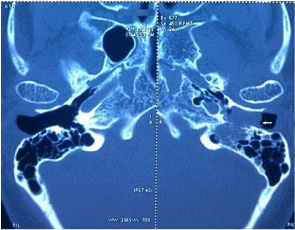
Figure 1: Contrast CT Scan of the temporal bones showing the mass in
external auditory canal.
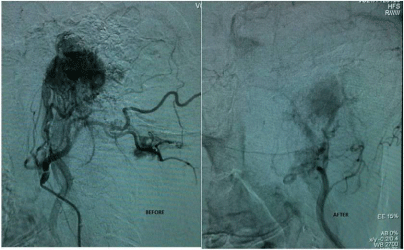
Figure 2: DSA pictures (a) Tumour blush before embolization, (b) Showing
loss of tumour blush after embolization of feeders.
The histopathological study on Hematoxylin and Eosin stain on 200 X magnification showed partially squamous epithelium covered connective tissue, and occasional entrapped bone spicules showing an infiltration in the sub-epithelial / interstitial stroma by a neoplasm composed of expansile lobules and alveolar nests of cells with central moderately atypical nuclei and clear cytoplasm suggestive of a clear cell carcinoma of the kidney (Figure 3). Immunohistochemistry was done for confirmation of the type of the tumour. Immunohistochemistry panel included Cytokeratin (CK), Vimentin, Cluster of Differentiation 10 (CD10), RCC antigen, S-100 protein and Carcino Embryonic Antigen (CEA). Tumor cells were positive for CK (Figure 4a), Vimentin (Figure 4b), CD10 (Figure 4c) and RCC antigen (Figure 4d). Tumor cells show intra-cytoplasmic glycogen (PAS - positive, PAS with D - sensitive). Tumor cells were negative for S-100 protein and CEA. The final diagnosis was clear cell carcinoma with possibility of metastatic renal cell carcinoma. She was referred to the oncology department where in a Positron Emission Tomography (PET) Scan was done which showed heterogeneously enhancing Fluoro Deoxy Glucose (FDG) avid hypodense lesion arising from lower pole of right kidney along with FDG avid lytic destructive lesions in left mastoid antrum, left external auditory canal and FDG avid lytic lesions in 3rd sacral (S3) and 2nd lumbar (L2) vertebra (Figure 5). The case was discussed in the tumour board and was advised palliative radiotherapy of 4500 Centi Gray (cGy) to lumbosacral region and left mastoid antrum over six weeks. She was then advised to be maintained on oral chemotherapy with Tab. Pazopanib hydrochloride. Repeat PET CT Scan at the end of six months showed complete metabolic resolution of FDG avid lesion in right kidney with development of necrosis with a significant decrease in FDG avidity of skeletal lesions (Figure 6). She is now being treated with oral chemotherapy alone by daily administration of Tab. Pazopanib hydrochloride 400mg for the past 24 months.
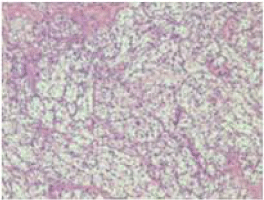
Figure 3: Histopathological picture showing the features of a clear cell
carcinoma.
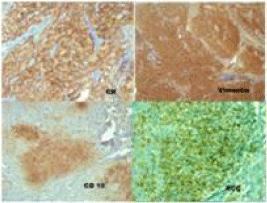
Figure 4: Immunohistochemistry pictures showing positive stain for (a) CK,
(b) Vimentin, (c) CD 10, (d) RCC antigen.
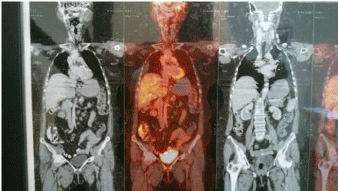
Figure 5: PET scan picture showing lesions in the right kidney, vertebra and
mastoid.
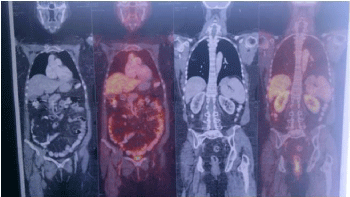
Figure 6: Post Chemoradiation PET Scan picture showing reduced FDG
avidity in the lesions.
Informed consent has been obtained from the patient to discuss and publish all relevant case facts and corroborative evidences for the purpose of scientific advancement in any journal.
Discussion
Metastases to the head and neck in Renal Cell carcinoma (RCC) are rare. Sountoulides et al. [2] have given a detailed description of unusual presentations and uncommon metastatic sites in renal cell carcinoma in regions like head and neck, orbit, parotid gland, nasal and paranasal sinus cavities, tongue and tonsils, thyroid gland, heart, skin, ovaries, uterus, testis, muscles and joints. RCC is next to only lung and breast carcinomas in the third position in the list of cancers that spread to the head and neck. Incidence in the fifth or sixth decade of life is more common and males are more afflicted than females [3]. Metastatic lesions can propagate to several areas in the body; the frequently affected organs being lungs (50-60%), long bones (30- 40%), hepatobiliary tract (30-40%) and brain (5%) [4]. Head and neck metastatic lesions in RCC have been found in the thyroid glands, paranasal sinuses, nasal cavities, skin and laryngeal cartilages in about 14 to 16% of cases. Isolated head and neck metatastatic lesions alone occur only in 1% of patients with primary RCC [5]. However, renal cell carcinoma presenting solely as a vascular mass in the ear canal is unprecedented. There is no mention in literature about this extremely rare presentation of metastatic renal cell carcinoma.
The pattern of spread of the metastatic lesions of RCC has been well-described by Boles and Cerny [5]. Vascular routes of distribution to the head and neck include the arteries, veins and lymphatics. The rich plexus of veins between the vertebral, prevertebral and epidural circulations acts as a corridor for the propagation of the neoplasm. Usual manifestations of RCC in the head and neck regions are visibly enlarged lump, nasal bleeding, tenderness in the face or sinuses and nasal blockage, which again depends on the exact location of tumour and depth of invasion [6].
RCC has been found to be resistant to treatment by radiotherapy. Nevertheless, radiotherapy can be recommended for the purpose of palliative treatment in advanced metastatic disease. Good responses have been found with 4500 cGy of radiotherapy treatment when given over a duration of four and a half weeks. This shows that radiotherapy can be useful in titrated doses for achieving palliative results [7]. Surgical attention to the primary tumour by nephrectomy in metastatic cases of RCC is recommended only for the purpose of improving the quality of life or local symptoms [6]. It does not cause any natural regression of the tumour growth. Although metastatic RCCs of the head and neck are rare, the otolaryngologist must have a high degree of suspicion when evaluating solitary unexplained atypical lesions in the head and neck. Presentations like our case where in there is nothing to suggest any background of renal cell carcinoma are rare and are often not suspected on initial diagnosis. This case report therefore adds another unprecedented variation in the pattern of metastatic spread of RCC to the head and neck. To date, there is no description in literature of any case of metastatic renal cell carcinoma presenting solely as a vascular mass of the external auditory canal.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006; 56: 106-130.
- Sountoulides P, Metaxa L, Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: a review of case reports. J Med Case Rep. 2011; 5: 429.
- Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996; 335: 865-875.
- Ritchie AW, Chisholm GD. The natural history of renal carcinoma. Semin Oncol. 1983; 10: 390-400.
- Boles R, Cerny J. Head and neck metastases from renal carcinomas. Mich Med. 1971; 70: 616-618.
- Huang H-C, Chang K-P, Chen T-M, Wu K-F, Ueng S-H. Renal Cell carcinoma metastases in the head and neck. Chang Gung Med J. 2006; 29: 59-65.
- Onufrey V, Mohiuddin M. Radiation therapy in the treatment of metastatic renal cell carcinoma. Int J Radiat Oncol Biol Phys. 1985; 11: 2007-2009.