
Case Report
Austin J Otolaryngol. 2015; 2(9): 1063.
Isolated Rhino-Orbital-Cerebral Mucormycosis. A Case Report and Literature Review
Barion U*, Cazzador D, Emanuelli E, Borsetto D, Alexandre E, Volo T, Pedruzzi B, Faccioli C, Prosenikliev V and Martini A
Department of Neuroscience, Padua University, Italy
*Corresponding author: Barion U, Department of Neuroscience, Operative Unit of Otolaryngology, Padua University, 35100, Italy
Received: August 25, 2015; Accepted: November 19, 2015; Published: November 21, 2015
Abstract
Mucormycosis represents an exceedingly rare invasive fungal infection, rapidly fatal if not promptly recognized and treated. Zygomycetes cause clinically relevant infections especially in elderly, diabetic or immunocompromised patients. Rhino-orbital-cerebral mucormycosis (M-ROC) is the most common form of zygomycosis. Despite therapeutic progresses, M-ROC is still characterized by high mortality rates, estimated between 35 and 66%. The objectives of this report are to describe a case of M-ROC in a 60-year-old woman presenting with asthenia and mild persistent fever and to review the literature related to the subject. After medical and surgical treatment, the patient is alive at 32-months follow-up. Only a high suspect of the disease and the alert for subtle clinical signs may lead to the prompt identification of Mucormycosis and to adequate medical and surgical management, resulting in improvement of the already poor prognosis of such an invasive infection.
Keywords: Mucormycosis; Rhino-orbital-cerebral mucormycosis; Invasive fungal sinusitis; Diabetic ketoacidosis
Abbreviations
M-ROC: Rhino-orbital-cerebral Mucormycoses; DKA: Diabetic Ketoacidosis; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; ESS: Endoscopic Sinus Surgery; LAmB: Liposomal Amphotericin B; AmB: Amphotericin B
Case Presentation
A 60-year-old Caucasian woman presented to the Emergency Room of our Institution with severe asthenia and mild persistent fever. One week earlier she experienced poliuria and polidipsia associated with intensive productive cough and some isolated episodes of hemoptysis. She had a history of hypertension and grade III bronchial asthma, which last reported severe outbreak dated back to 30 days before. Due to her respiratory problem, the patient has been treated with oral corticosteroids for more than 2 years (which has been not regularly medically checked). Given the patient’s clinic and the laboratory findings, a diagnosis of diabetic ketoacidosis (DKA) and pulmonary infection was made and the patient was admitted to the Metabolic Disease Department of our Institution. The patient was treated with an antibiotic therapy (meropenem and Ampicillin/ sulbactam) associated with insulin and short-term injective steroid therapy.
Few days after the admittance, the patient complained of diplopia and numbness at the right side of the forehead skin. Fixed mydriasis and ptosis of the right eye were noticed at physical examination, thus the patient underwent ophthalmological evaluation, which reported a right rectus medialis muscle deficit. The fundoscopic eye exam showed a pale, atrophic papilla on the right side. The imaging - brain and head contrasted computed tomography (CT) scan and angiomagnetic resonance imaging (MRI) - showed a pansinusitis condition complicated with a right fronto-basal brain abscess and a right orbital abscess (Figure 1A,B). Nasal endoscopy found the presence of necrotic material, scabs and purulent exudates in the nasal cavity, suggesting the presence of an invasive fungal sinusitis (Figure 2).
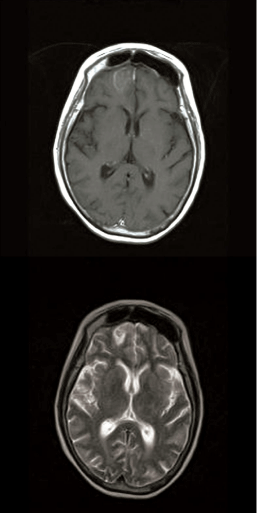
Figure 1: Preoperative T1-weighted axial MRI with contrast infusion (A),
and preoperative T2-weighted axial MRI (B) showing the right frontal brain
abscess.
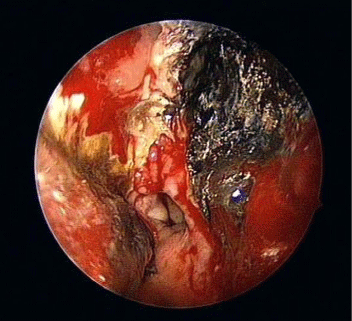
Figure 2: Intraoperative endoscopic finding in the left nasal fossa.
The patient was submitted to bilateral endoscopic sinus surgery (ESS) with bilateral orbital decompression and debridement of necrotic tissue material. No intra- or postoperative complications occurred. Intraoperative findings revealed the presence of necrotic crusty material in both nasal cavities and a wide necrosis of the nasal turbinates and septum. Mucosal tissue samples and scabs were collected for histological and microbiological examination. The neurosurgeon did not indicate surgical drainage of the brain abscess, recommending close observation and prophylactic anticonvulsant therapy with levetiracetam.
The confirmation of M-ROC has been reached through the histological and microbiological examination. The patient received antifungal therapy with intravenous liposomal amphotericin B (LAmB) dosed at 7 mg/Kg QD for 67 days. Thereafter, the injective therapy was replaced by oral intake of posaconazole dosed at 400 mg BID, which the patient continued for 8 months after the discharge. Patient comorbidities have also been treated, obtaining good glycemic compensation and resolution of the pulmonary infection. Fortyeighth days after the first intervention the patient was submitted to a revision of ESS, in order to remove bony sequestra of the sphenoethmoidal sinuses.
The outcome was favorable. The patient survived an invasive sinonasal fungal infection with brain and orbital extension, reporting a right eye amaurosis as the only sequela. At 32-month follow-up the nasal endoscopy (Figure 3) revealed well-aerated nasal cavities and sinuses covered by healthy mucosa. No evidence of infection relapse was identified, as confirmed by RMI scans, performed every 3 months after surgical treatment (Figure 4).
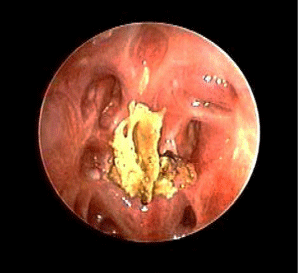
Figure 3: Nasal endoscopy performed 32 months after surgery showing the
neo-ethmoidal cavity.
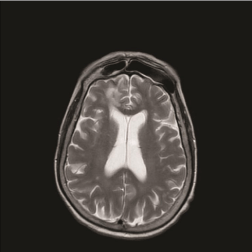
Figure 4: Postoperative T2-weighted axial MRI, at 32 months after surgical
treatment.
Discussion
Human mucormycosis was first described by Paltauf in 1885with the term of mycosis mucorina [1]. Mucormycosis represents an emerging, rapidly progressive, invasive life-threatening fungal infection [3]. The annual estimated incidence in France is in average 0.9 cases/million [3]. In the United States it amounts to 1.7 cases/ million [4], with a male to female ratio of 2:1. The age of onset ranges from 6 days to 75 years, with a mean of 38.8 years [5].
The etiological agent of mucormycosis belongs to the class of Zygomycetes (Mucorales order), being Rhizopusoryzae the most frequently isolated organism, responsible for about 70% of all infections, followed by Rhizopus rhizopodiformis and Rhizopus microspores [5-7].
The inhalation of sporangiospores from environmental sources (soil, decaying grass, leaf mold) represents the principle acquisition mode of the fungal elements [6].
The largest review on zygomycosis in the English literature underlines the rarity of the pathology. Only 929 cases have been collected from 1885 to 2005, reporting a prevalence of 39% in Sinonasal zygomycosis, being the rhino-cerebral infection the most common pattern of spreading. Mortality has remained effectively unchanged for over 50 years, after the introduction of amphotericin B. To date, overall mortality ranges between 35% and 66%, by reason of different underlying patients conditions [5].
Depending on host characteristics (immunosuppressive therapy, diabetes mellitus, immune system disorders - leukemia’s, lymphomas, solid tumors, neutropenia, and use of steroids, bone marrow transplantation, drugs injection and increased level of serum iron) and way of infection, mucormycosis may present itself in form of rhino-orbito-cerebral, pulmonary, gastrointestinal, cutaneous, or disseminated disease [7]. The status of the underlying pre-existing patient’s comorbidities and the timing of diagnosis are of prime importance in obtaining a good outcome. Diabetes mellitus type 1 and 2 are considered independent predictors for sinus zygomycosis presenting an odds ratio of 4.04 and 6.35 respectively [5]. Thus, between 33% and 70% of M-ROC cases occur in patients with DKA [6,7].
Angioinvasion is considered the hallmark of mucorine infection, with consequent thrombosis of blood vessels and tissue necrosis. Moreover, the diabetic microvascular disease, in addition to the delicate anatomical architecture of the sinonasal district, may result in tremendous tissue destruction [5,8].
Diagnosis of mucormycosis is often delayed due to the extremely different clinical presentation of the infection. Three clinical stages characterize M-ROC infection: limited sinonasal disease, limited rhino-orbital disease and rhino-orbito-cerebral disease. Some suspect-evoking signs have been proposed for each stage, such as mucopurulent or bloody rhinorrhea for stage I, diplopia for stage II, bloody nasal discharge and neuropsychiatric changes for stage III [8- 10].
Histopathological examination with hematoxylin and eosin stain, Gomori methenamine-silver or periodic acid-Schiff, shows characteristic broad, hyaline, ribbon-like, wide-angled branching, aseptate fungal hyphae, with areas of tissue necrosis and angioinvasion [6,9]. The imaging indicates the extension of the infection [7]. Moreover, in early stages of mucormycosis contrasted CT scan of the brain and facial bones reveals non-specific signs of sinus involvement and thickening of the mucosa [10]. Lack of enhancement of the superior ophthalmic vein or ophthalmic artery and internal carotid artery relates to vasculitis and thrombosis [11]. Cerebral involvement may be diagnosed by the presence of cerebral abscess, multiple infarctions areas or even cavernous sinus thrombosis [7,12,13]. Contrasted MRI is more sensitive than CT scan [14] in achieving an early diagnosis of intracranial or orbital infiltration. It also represents the gold standard in the follow-up period [7].
The multi-modality approach of treatment includes four critical factors needed for successful management of mucormycosis: (a) promptness of diagnosis, (b) appropriate antifungal therapy, (c) surgical management and (d) control of the underlying medical illnesses [7,8,12,15,16]. First of all hyperglycemia and metabolic acidosis (in particular DKA) have to be aggressively adjusted. Moreover, reduction or temporary discontinuation of corticosteroids or immunosuppressive agents should be considered, until infection is controlled [17].
The standard antifungal treatment consists of amphotericin B (AmB) infusion, increasingly dosed until reaching 1 mg/kg QD [18]. Antifungal treatment should be continued up to 10 - 12 weeks. The positive role of LAmB, compared to AmB, has been demonstrated in terms of reduced nephrotoxicity [8,12]. Furthermore, LAmB therapy is associated to a higher survival rate compared to AmB [18] and has shown better tissue diffusion in the central nervous system in animal models [19]. New antifungal agents have recently being tested. Posaconazole is indicated as a second line drug for patients with AmB treatment failure or in case of AmB in tolerance [20,21]. Posaconazole seems also to be well tolerated in immunocompromised patients, being only gastrointestinal symptoms and headache the most frequently reported adverse effects [22,23].
Because of the aggressive nature of the disease, medical therapy alone appeared being often inadequate in infection control. A multivariate analysis clearly demonstrates how antifungal therapy and surgery are independently associated with a decreased risk of mortality [5]. Surgical management becomes crucial and must not be delayed [6]. Surgery consists in the debridement of the necrotic tissues. As in the reported case, more than one surgical session may be necessary. More extensive surgical measures such as orbital decompression or exenteratioorbitae are required in order to obtain a life-saving control of the infection [24]. In the presented case, brain abscess has been treated conservatively based on the good response to medical therapy. In literature, to date, there is no defined treatment protocol regarding invasive fungal brain abscesses [17]. According to the protocol of our Institution, brain abscesses located in the frontal-basal region, which are <3 cm in size and seem to be directly correlated with a paranasal frontal sinus infection, primarily receive medical therapy. A strict RM follow-up imaging is required in order to state the response to conservative therapy. When it fails, neurosurgical intervention is recommended.
Conclusion
The spontaneous evolution of M-ROC is always fatal. Despite recent therapeutic progresses, the prognosis of M-ROC remains poor, depending on how promptly the diagnosis is made, how fast the antifungal treatment associated to surgical treatment are given and how rapidly the underlying disease is managed [7,8]. The main reported factors for poor prognosis seem to be diabetes mellitus, metabolic acidosis, prolonged corticosteroid therapy and chronic kidney disease. As concerns M-ROC, the overall survival rate depends on infection localization. In particular: it is of 15% in cerebral presentations, and rises to 50% and 75% in rhinoorbital presentations, and when no other disorders are associated, respectively [7-9].
Being a delayed treatment an independent factor for poor outcome [9], a successful result is based on a high level of clinical suspect of the disease and its associated risk factors, thus making it possible to obtain early diagnosis and appropriate combined surgicalmedical management.
References
- Paltauf A. Mycosis mucorina: Ein Beitrag zur Kenntniss der menschlichen Fadenpilzerkrankungen. Virchows Arch.1885; 102: 543-564.
- Castelnuovo P, Gera R, Di Giulio G, Canevari FR, Benazzo M, Emanuelli E, et al. [Paranasal sinus mycoses]. Acta Otorhinolaryngol Ital. 2000; 20: 6-15.
- Bitar D, Van Cauteren D, Lanternier F, Dannaoui E, Che D, Dromer F, et al. Increasing incidence of zygomycosis (mucormycosis), France, 1997-2006. Emerg Infect Dis. 2009; 15: 1395-1401.
- Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin Infect Dis. 1998; 27: 1138-1147.
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005; 41: 634-653.
- Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000; 13: 236-301.
- Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005; 18: 556-569.
- Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D'Souza O. Rhino-orbito-cerebral mucormycosis. A retrospective analysis of clinical features and treatment outcomes. Indian J Ophthalmol. 2003; 51: 231-236.
- Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis. 2012; 54 Suppl 1: S55-60.
- Dhiwakar M, Thakar A, Bahadur S. Improving outcomes in rhinocerebral mucormycosis--early diagnostic pointers and prognostic factors. J Laryngol Otol. 2003; 117: 861-865.
- Bae MS, Kim EJ, Lee KM, Choi WS. Rapidly Progressive Rhino-orbito-cerebral Mucormycosis Complicated with Unilateral Internal Carotid Artery Occlusion: A Case Report. Neurointervention. 2012; 7: 45-49.
- Talmi YP, Goldschmied-Reouven A, Bakon M, Barshack I, Wolf M, Horowitz Z, et al. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngol Head Neck Surg. 2002; 127: 22-31.
- Koc Z, Koc F, Yerdelen D, Ozdogu H. Rhino-orbital-cerebral mucormycosis with different cerebral involvements: infarct, hemorrhage, and ophthalmoplegia. Int J Neurosci. 2007; 117: 1677-1690.
- Fatterpekar G, Mukherji S, Arbealez A, Maheshwari S, Castillo M. Fungal diseases of the paranasal sinuses. Semin Ultrasound CT MR. 1999; 20: 391-401.
- Chayakulkeeree M, Ghannoum MA, Perfect JR. Zygomycosis: the re-emerging fungal infection. Eur J Clin Microbiol Infect Dis. 2006; 25: 215-229.
- Anselmo-Lima WT, Lopes RP, Valera FC, Demarco RC. Invasive fungal rhinosinusitis in immunocompromised patients. Rhinology. 2004; 42: 141-144.
- Gamaletsou MN, Sipsas NV, Roilides E, Walsh TJ. Rhino-orbital-cerebral mucormycosis. Curr Infect Dis Rep. 2012; 14: 423-434.
- Spellberg B, Ibrahim A, Roilides E, Lewis RE, Lortholary O, Petrikkos G, et al. Combination therapy for mucormycosis: why, what, and how? Clin Infect Dis. 2012; 54: S73-78.
- Groll AH, Giri N, Petraitis V, Petraitiene R, Candelario M, Bacher JS, et al. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J Infect Dis. 2000; 182: 274-282.
- Enoch DA, Aliyu SH, Sule O, Lewis SJ, Karas JA. Posaconazole for the treatment of mucormycosis. Int J Antimicrob Agents. 2011; 38: 465-473.
- van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006; 42: e61-65.
- Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007; 356: 348-359.
- Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007; 356: 335-347.
- Eicken Jv, Preyer S, Wilhelm H. [Potentially fatal orbital disorder]. Klin Monbl Augenheilkd. 2004; 221: 948-952.