
Case Report
Austin J Otolaryngol. 2015; 2(9): 1065.
Dermatofibrosarcoma Infiltrating the Parotid Gland: Case Report and Literature Review
De Liz AA*, Cardoso DB, Nery dos Passos Martins DA and Lunardi FA
Department of Otolaryngology and Head and Neck surgery, University of São Paulo, Brazil
*Corresponding author: Alcebiades Alves De Liz, Department of Otolaryngology and Head and Neck surgery, Hospital de Reabilitação de Anomalias Craniofaciais, Universidade de São Paulo, Bauru–SP, Postal code: 17.012-900, Brazil
Received: October 30, 2015; Accepted: December 20, 2015; Published: December 24, 2015
Abstract
Introduction: Primary dermatofibrosarcoma protuberans (DFSP) of the salivary gland is extremely rare.
Case Presentation: A 37-year-old female complained of a 5-year, slowgrowing lump in the right parotid region without any other sign or symptom. At examination there was a firm, non-movable nodule on the right parotid gland. The ultrasound/Doppler showed a well-vascularized solid nodule with ill-defined borders on the inferior edge of the parotid. A total parotidectomy was performed. Sections revealed an ill-defined, non-encapsulated nodule measuring 2.5 x 2.0 x 1.3 cm. The histopathological diagnosis coupled with immunohistochemistry was consistent with DFSP.
Conclusion: We report a rare case of DFSP infiltrating the parotid gland. Whether it represents a primary lesion or a subcutaneous tumor infiltrating the parotid gland, this tumor presented clinically as a parotid lump and diffusely infiltrated this gland. Treatment modalities and prognosis are not well-defined due to the scarcity of cases described in the literature.
Introduction
Tumors of mesenchymal origin can occur in any part of the body but are rare in salivary glands [1]. Malignant mesenchymal tumors are seldom encountered in major salivary glands and soft tissue sarcomas account for only 0.3–1.5% of all salivary gland neoplasms [2]. Moreover, approximately 5% of all primary salivary gland tumors are of mesenchymal origin [3], but only 0.3–1.5% are malignant [4].
Dermatofibrosarcoma Protuberans (DFSP) was first described in 1924 by Darier and Ferrand as a ‘‘progressive and recurring dermatofibroma””, and is a low–grade malignant tumor that typically presents on the trunk and proximal extremities as a slow–growing, painless, firm, cutaneous plaque. However, unusual sites for DFSP, including the vulva and parotid gland, have been reported [5].
Primary DFSP of the salivary gland is extremely rare and to the best of our knowledge only 4 cases have been reported in the English literature [1].
Case Presentation
A 37-year-old female complained of a 5-year, slow-growing lump in the right parotid region without any other sign or symptom. Patient had no previous history of surgery or other relevant disease. At examination there was a firm, non-movable nodule on the right parotid gland with a slight skin retraction without inflammatory sings. The ultrasound/Doppler, bi-dimensional method, with a linear dynamic equipment (7.5 MHz) showed a well-vascularized solid nodule with ill-defined infiltrative borders at the inferior edge of the parotid measuring approximately 2.0 x 2,0 x 1,5 cm (Figure 1). No skin involvement was seen. A diagnosis of parotid tumor was performed and carcinoma was the main hypothesis. A total parotidectomy was performed.

Figure 1: A: US.
Gross examination showed a brownish parotid measuring 5.0 x 4.5 x 1.5 cm, with an irregular and lobulated surface. Sections revealed an ill-defined, non-encapsulated nodule measuring 2.5 x 2.0 x 1.3 cm, with a whitish, solid and infiltrative surface.
Histopathologically the tumor was entirely composed of spindle cells arranged in short fascicles with a storiform pattern. The tumor was well-vascularized with an infiltrative pattern that dissected normal residual glands and adipose tissue (Figure 1). The spindle cells had a uniform appearance with ill-defined borders, mild to moderate nuclear atypia and conspicuous mitotic figures (Figure 2). Neither necrosis nor atypical mitotic figures were present. Collagen was scattered within the lesion but thick collagen bundles were not seen.
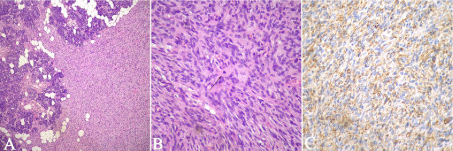
Figure 2: 2A: The spindle cell tumor diffusely infiltrates the residual salivary
gland tissue in a vague storiform pattern [hematoxylin-eosin (H&E), x40].
Figure 2B: High power view showing that tumor was composed of spindle
cells with moderate nuclear atypia. Mitoses were rare but conspicuous
through the tumor (arrow) (H&E, x400). Figure 2C: Immunohistochemistry
for CD34 showing that tumoral cells were strong and diffusely positive for
this marker.
With immunohistochemistry, the tumoral cells exhibited diffuse and strong immunoreactivity for Vimentin, CD34 and Bcl-2 (Figure 2) and were negative for S-100, EMA, Desmin and AE1AE3. The diagnosis was consistent with DFSP.
The patient received radiotherapy and remained free of recurrence or metastasis after 5 years of follow-up.
Discussion
The most common primary benign parotid tumor, which includes pleomorphic adenoma and warthin tumor, are usually firm and mobile lumps that may attenuate the overlying skin or mucosa [5]. However, our patient presented a firm but non-movable infiltrative nodule and malignancy was suspected in the first occasion. Therefore, Adenoid cystic carcinoma and mucoepidermoid carcinoma were the main differential diagnosis both on clinical and US examination.
The incidence of sarcomas of the major salivary gland appears to be about one tenth of benign mesenchymal tumors [3], and small series or individual cases of various sarcomas have been described [1].
The major differential diagnoses of a primary salivary gland sarcoma are metastases from a sarcoma at a different primary site [6] and the mesenchymal component of a carcinosarcoma [7]. Our patient didn”t have a history of another sarcoma and physical exam revealed no tumor elsewhere. Moreover, the entire specimen was included for histopathological analysis and no component of carcinoma or other differentiation was found.
Another criterion that must be considered is that gross and microscopic appearances must support primary origin rather than invasion from adjacent soft tissue [1]. Our patient complained of an increase in volume of the parotid gland for a long time without any other skin lesion. Although we cannot confidently state that the tumor originated from the parotid stroma rather than from the deep subcutaneous tissue, our patient presented clinically with a parotid tumor. Furthermore, DFSP with a symptom of parotid enlargement has been poorly reported and been considered to be primary from the parotid. Lee et al. [8] reported a case that they called primary parotid DFSP in which the tumor was completely enclosed within a fibroadipose capsule of the parotid gland.
A primary skin DFSP would present with a visible lump in the skin. In our patient, the tumor was a poorly-defined nodule located deep on the gland without noticeable skin involvement. Cases of DFSP infiltrating other salivary glands are limited in the literature.
In the differential histopathological diagnosis, solitary fibrous tumor (SFT), benign fibrous histiocytoma (FH), schwannoma, myoepithelioma, and other spindle cell sarcomas have to be considered. SFT is the most difficult entity to distinguish from DFSP because of the somewhat similar histologic findings and overlapping immunohistochemical staining results. SFT is characterized by a variety of growth patterns. There is frequently a mixture of spindle cell areas with a distinct hemangiopericytic vasculature and foci of sclerosis or myxoid changes. DFSP, unlike solitary fibrous tumor, shows remarkable uniformity, a lack of the hemangiopericytic pattern, has a distinct storiform pattern around an inconspicuous vasculature [8] and an infiltrative border [1]. Additionally, due to overlapping immunohistochemical results for DFSP and SFT, caution is required in the differential diagnosis [8].
Benign FH may resemble DFSP but it is usually negative for CD34 and Bcl-2 expression. Schwannoma contains Antoni A and B areas and it is S-100 protein positive, something not seen in DFSP. Other spindle cell tumors of the parotid gland in the differential diagnosis include spindle cell myoepithelioma, malignant peripheral nerve sheath tumor, fibrosarcoma and malignant FH. A proper use of immunohistochemical markers in conjunction with the characteristic light microscopic features helps to differentiate these tumors from DFSP [8].
In general, head and neck sarcomas are rare and the paucity of studies about their management and outcome represents this rarity [9]. The standard follow-up for skin DFSP includes examination every 6 months in the first 3 years and annually after this period [1], and in our point of view this should be the minimum for a patient with parotid DFSP. There are several histological subtypes of sarcomas that present with a variety of clinical characteristics and many often require treatment with a combination of surgery, radiotherapy, and chemotherapy [9]. For salivary glands sarcomas, the most successful treatments are wide surgical excision or surgery combined with radiation [5].
The 5-year survival rate for head and neck sarcomas is approximately 50% [9,10]. Most authors agree that the same prognostic factors– grade, size, and depth-apply to sarcomas no matter where they arise. In the head and neck, however, local recurrence has more significant consequences because of the difficulty of subsequent management [9]. In general, salivary gland sarcomas are aggressive neoplasms with recurrences in about 40-64%, haematogenic metastasis and mortality rates ranging from 36-64% [5]. The prognosis for parotid DFSP is not clear due to the scarcity of cases reported in the literature.
Conclusion
In conclusion, we report a rare case of DFSP infiltrating the parotid gland. Whether it represents a primary lesion or a subcutaneous tumor infiltrating the parotid, that could not be distinguished by the histopathological exam, this tumor presented clinically as a parotid lump and diffusely infiltrated this gland. Treatment modalities and prognosis are not well-defined due to the scarcity of cases described in the literature.
References
- Cho KJ, Ro JY, Choi J, Choi SH, Nam SY, Kim SY. Mesenchymal neoplasms of the major salivary glands: clinicopathological features of 18 cases. Eur Arch Otorhinolaryngol. 2007; 265: 47-56.
- Kang J, Levinson JA, Hitti IF. Leiomyosarcoma of the parotid gland: a case report and review of the literature. Head Neck. 1999; 21: 168-171.
- Seifert G, Oehne H. [Mesenchymal (non-epithelial) salivary gland tumors. Analysis of 167 tumor cases of the salivary gland register]. Laryngol Rhinol Otol (Stuttg). 1986; 65: 485-491.
- Krüger S, Sommer K. Leiomyosarcoma of the parotid gland: a case report. Eur Arch Otorhinolaryngol. 2006; 263: 951-954.
- Weiss SW, Goldblum JR. Enzinger and Weiss”s soft tissue tumors. Mosby, St. Louis. 2001.
- Saiz AD, Sachdev U, Brodman ML, Deligdisch L. Metastatic uterine leiomyosarcoma presenting as a primary sarcoma of the parotid gland. Obstet Gynecol. 1998; 92: 667-668.
- Kwon MY, Gu M. True malignant mixed tumor (carcinosarcoma) of parotid gland with unusual mesenchymal component: a case report and review of the literature. Arch Pathol Lab Med. 2001; 125: 812-815.
- Lee OJ, Pi DY, Jo DH, Cho KJ, Kim SY, Ro JY. Dermatofibrosarcoma Protuberans of the Parotid Gland - A Case Report. The Korean Journal of Pathology. 2004; 38: 276-279.
- Singh RP, Grimer RJ, Bhujel N, Carter SR, Tillman RM, Abudu A. Adult head and neck soft tissue sarcomas: treatment and outcome. Sarcoma. 2008; 2008: 654987.
- Bentz BG, Singh B, Woodruff J, Brennan M, Shah JP, Kraus D. Head and neck soft tissue sarcomas: a multivariate analysis of outcomes. Ann Surg Oncol. 2004; 11: 619-628.