
Research Article
Austin J Otolaryngol. 2016; 3(1): 1069.
Otolith Dysfunction can Affect Head Stability during Gait
Aboshanif M, Itasaka Y, Omi E, Koizumi K and Ishikawa K*
Department of Otorhinolaryngology, Head & Neck Surgery, Akita Graduate School of Medicine, Akita, Japan
*Corresponding author: Kazuo Ishikawa, Department of Otorhinolaryngology, Head & Neck Surgery, Akita Graduate School of Medicine, 1-1-1 Hondo, Akita 010- 8543, Japan
Received: January 27, 2016; Accepted: February 25, 2016; Published: February 29, 2016
Abstract
We study in detail the influence of Otolith organ dysfunction on head stability during locomotion. Participants were 20 patients (mean age, 54.0±11.6 years; mean height, 160.9±8cm) with unilateral Acoustic Neuroma (AN), and 9 age- and height-matched controls (mean age, 60.1±8.5 years; mean height, 162.7±8.1cm). All participants underwent measurement of ocular Vestibular Evoked Myogenic Potential (oVEMP) and cervical VEMP (cVEMP). Subjects were asked to walk freely with eyes open and closed while head movements were analyzed using a 3-dimensional motion analysis system. With regard to VEMP test results, oVEMP only, cVEMP only and both were abnormal in 3, 5 and 9 patients, respectively. Three patients showed normal results for both VEMPs. Compared with controls, horizontal head movement was greater in patients with abnormal oVEMP during eyes open and closed conditions and in patients with abnormal results for both VEMP tests during the eyes closed condition, while head movement in the pitch plane during walking with eyes open and closed, and in the roll plane with eyes closed was greater in patients with abnormal cVEMP or abnormal both VEMP tests. We conclude that, head stability in the horizontal plane during locomotion is affected by utricular dysfunction, while stability in the pitch and roll planes is affected by saccular dysfunction.
Keywords: Head movement; Locomotion; Three-dimensional motion analysis; VEMP; Utricle; Saccule
Abbreviations
AN: Acoustic Neuroma; OVEMP: Ocular Vestibular Evoked Myogenic Potential; CVEMP: Cervical Vestibular Evoked Myogenic Potential; SCM: Sternocleidomastoid Muscle; EMG: Electromyography
Introduction
Almost all Acoustic Neuromas (ANs) arise from the superior or inferior vestibular nerve, and few arise from the cochlear nerve [1]. The two Otolith organs (the utricle and saccule) sense linear acceleration of the head in the horizontal and vertical planes, respectively [2]. Also, afferents from the utricular macula course within the superior division of the vestibular nerve together with a small contingent from the hook region of the saccular macula, and the bulk of the fibers from the saccular macula project into the inferior vestibular nerve [3,4]. Thus, cases with AN could result in some dysfunction to the superior or inferior vestibular nerves, or both. Such functional disorder could affect gait control systems to some extent [5]. Recently, Ocular Vestibular Evoked Myogenic Potential (oVEMP) and Cervical Vestibular Evoked Myogenic Potential (cVEMP) tests were shown to be able to reveal utricular and saccular function independently [6]. In a previous study, we proved that dysfunction of the vestibular nerve in patients with AN could affect head stability during walking [7]. The present study examined in detail the effect of Otolith organ dysfunction on head stability in all planes of movement during locomotion using our 3-dimensional motion analysis system.
Patients and Methods
Twenty patients with unilateral AN diagnosed by neurootological examinations and MRI with contrast enhancement were enrolled in this study. The mean age of patients was 54.0±11.6 years and mean height was 160.9±8.2cm. The tumor was intra-canalicular in 5 patients, <10mm in 6 patients, 10-20mm in 5 patients and 20- 32mm in 4 patients.
Nine age- and height-matched healthy subjects served as controls. Mean age was 60.1±8.5 years and mean height was 162.7±8.1cm. All patients underwent monitoring of oVEMP and cervical VEMP, and were asked to walk freely with eyes open and closed for distances of about 5 m while head movements were analyzed using a 3-dimensional motion analysis system. The study was conducted according to the tenets of the Helsinki Declaration. All subjects signed written informed consent prior to participation in this study.
Testing of otolith organ function
As an initial preparation for testing, impedance of the electrodes was reduced by wiping the skin carefully with alcohol-moistened cotton and surface electrodes were coated with conductive paste. Recording was performed using Navigator.PRO (Bio-Logic Systems Corporation, Illinois, US).
cVEMP
All participants were asked to raise their head to achieve maximal activation of the Sterno Cleidomastoid Muscle (SCM) on both sides. Active electrodes were placed on the middle third of the SCM, reference electrodes were placed on the upper part of the sternum and the ground electrode was placed at the center of the forehead. Surface Electromyography (EMG) was recorded for a series of 95-dB click stimuli delivered through an earphone at a frequency of 5Hz, and was band pass-filtered (10-1500Hz). Analysis time was 53.3ms, and responses to 100 stimuli were averaged. Mean peak latencies of the two early waves p13 and n23 (normal p13 latency, 13.7±1.0 ms; n23 latency, 23.0±1.8 ms), peak-to-peak (p13-n23) amplitude and asymmetry ratio (normally 15.9±8.4%) between both sides were measured. The first positive peak of VEMP was defined as p13, and the first negative peak following p13 as n23. Absence of a meaningful waveform with p13 and n23 was defined as “no response”.
The study was repeated for both sides twice, and values for latency and amplitude were calculated as means. Cases of absent response, asymmetry ratio >32.7% or elongation of p13 and n23 latencies (>15.7ms or >26.6ms, respectively) were considered abnormal.
oVEMP
Active, reference, and ground electrodes were placed on the lower eyelid, 2cm below and on the forehead, respectively. All cases were requested to look upwards to activate the inferior oblique muscle. Meanwhile, 95-dB click stimuli were delivered using an earphone to the contralateral ear at a frequency of 5Hz for duration of 53.3s, and 100 responses were averaged. Filter setting was 3-500Hz. The first negative and positive responses were designated as n1 and p1 waves, respectively. Latencies of n1 (10.5±1.1ms) and p1 (15.9±1.3ms), peakto- peak (n1-p1) amplitude and asymmetry ratio between both sides (normally 8.7±6.4%) were measured. The study was repeated 3 times on both sides, and mean values were calculated. Cases of no response, asymmetry ratio >21.5% or elongation of n1 and p1 latencies (>12.7ms, >18.5ms, respectively) were considered abnormal.
Analysis of head movement during locomotion
Three-dimensional head movement analysis was performed using our 3-dimensional motion analysis system. Reflective markers were placed on each side of the body at the following sites: shoulder, elbow, wrist, knee, heel, and toe. Another three markers were placed on the head at the forehead, vertex and inion. Subjects were asked to walk freely for a distance of about 5m with the eyes open or closed, and then to turn around and return to the starting position.
Three-dimensional gait analysis was performed in a 2meters cubic space set at the center of the pathway. In this study, analysis was focused on head movement during gait. Variables were thus head movements in the up-down and horizontal planes measured in centimeters, as well as the angles of roll, pitch and yaw movement. One-way ANOVA with post hoc analysis was employed for statistical analysis. Values of P<0.05 were considered statistically significant.
Results
VEMP test results
AN patients were grouped into 4 categories according to VEMP test results: those with normal results for both VEMP tests (3 cases); those with abnormal results for both VEMP tests results (9 cases); those with an abnormal oVEMP result, but a normal cVEMP (3 cases); and those with an abnormal cVEMP result, but normal oVEMP result (5 cases). As for caloric test results, most cases showed canal paresis, except for two cases from the group with normal results for both VEMPs, one case from the only cVEMP normal group and one case from the group with both VEMPs abnormal (Tables 1, 2).
Tests
Head movement
oVEMP(?)
cVEMP(?)
P value
oVEMP(?)
cVEMP(?)
P value
oVEMP(?)
cVEMP(?)
P value
oVEMP(?)
cVEMP(?)
P value
Up-down (cm)
Eyes-open
2.8±0.7
-
2.6±0.7
-
3.1±1.0
-
2.7±0.5
-
Eyes-closed
2.8±0.6
-
2.9±0.9
-
3.3±1.0
-
2.8±0.6
-
Horizontal (cm)
Eyes-open
5.8±1.5
-
7.5±2.5
0.0269
6.4±1.6
-
6.1±1.6
-
Eyes-closed
6.8±0.9
-
8.0±2.1
0.0223
6.7±2.2
-
8.2±2.4
0.0090
Table 1: Results of head movement in up-down and horizontal planes in acoustic neuroma groups when compared to the control group.
Tests
Head movement
oVEMP(?)
cVEMP(?)
P value
oVEMP(?)
cVEMP(?)
P value
oVEMP(?)
cVEMP(?)
P value
oVEMP(?)
cVEMP(?)
P value
Yaw (degree)
Eyes-open
3.6±1.6
-
3.1±0.3
-
4.3±11
0.0103
3.5±0.9
-
Eyes-closed
2.8±0.1
-
3.2±0.6
-
3.6±1.0
-
3.8±1.0
-
Pitch (degree)
Eyes-open
4.4±1.5
-
4.1±1.1
-
6.0±2.2
0.0422
5.6±1.5
0.0176
Eyes-closed
3.3±1.4
-
3.1±1.5
-
5.7±0.9
0.0001
5.0±1.7
0.0111
Roll (degree)
Eyes-open
3.9±1.2
-
5.4±1.4
-
5.9±2.1
5.2±1.6
Eyes-closed
4.2±1.7
-
3.0±1.7
-
5.4±1.0
0.0236
5.3±0.8
0.0083
Table 2: Results of head movement in yaw pitch and roll planes in acoustic neuroma groups when compared to the control group.
Head movement analysis during locomotion
Analysis of head movement during locomotion was performed with eyes open and closed. Move-tr/3D software (Library co., Ltd, Tokyo) was used to analyze head movements in different directions. Regarding head movement (measured in centimeters) in the updown direction, no significant difference was seen between the control and AN cases as a whole. Likewise, no significant difference was seen among AN categories under gait with eyes open and closed (Figure 1A). During the eyes-closed condition, head movement (measured in centimeters) in the horizontal plane was significantly greater in AN cases (p=0.0130), AN cases with abnormal oVEMP test (p=0.0223) and AN cases with abnormal results for both VEMP tests (p= 0.0090) than in the control group (Figure 1B). During the eyes-open condition, head movement in the horizontal plane was also greater in AN cases with abnormal oVEMP test and normal cVEMP test (p= 0.0269) than in the control group.
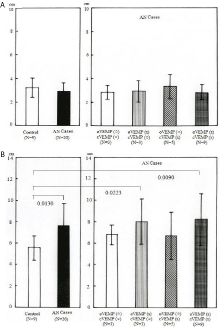
Figure 1: Head movement analysis. Results of head movement during gait
with eyes closed. A) Up-down movement shows no significant difference
between the control and AN groups, nor among AN categories. B) Horizontal
movement is significantly greater in the AN group, AN cases with abnormal
oVEMP and in AN cases with abnormal VEMP than in the control group.
Regarding head movement (measured in degrees) in the yaw plane, head movement with eyes open but not with eyes closed was significantly greater in AN cases with abnormal results for cVEMP and normal oVEMP (p=0.0103) than in the control group. No significant difference was seen in head movement between the control and AN groups. There was also no significant difference among AN categories (Figure 2). Conversely, in the pitch plane, head movement (measured in degrees) with eyes closed was significantly greater in AN cases (p=0.0231), AN cases with abnormal results for both VEMP tests (p=0.0111) and AN cases with abnormal cVEMP (p=0.0001) than in the control group. Head movement in that plane was also significantly greater in AN cases with abnormal cVEMP than in AN cases with normal results on both VEMP tests (p=0.0445) and AN cases with abnormal oVEMP test (p=0.0293) (Figure 3A), while head movement during the eyes-open condition was significantly greater in AN cases with abnormal cVEMP and abnormal both VEMP test results (p= 0.0422, 0.0176) respectively.

Figure 2: Results of head movement analysis in the yaw plane during gait
with eyes closed. No significant difference is apparent between the control
and AN groups, or among AN categories.
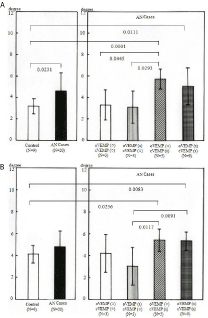
Figure 3: Results of head movement in A) The pitch plane and B) The roll
plane. Head movement was significantly greater in AN cases with abnormal
results for both VEMPs and AN cases with abnormal cVEMP during gait with
eyes closed compared to the control group.
Analysis of head movement in the roll plane during the eyes-closed condition showed that head movement (measured in degrees) was significantly greater in AN cases with abnormal results for both VEMP tests (p=0.0083) and AN cases with abnormal cVEMP test results (p=0.0236) compared to the control group. Also, head movement in AN cases with abnormal cVEMP groups was significantly greater than in AN cases with abnormal oVEMP (p=0.0091 and p=0.0117, respectively) (Figure 3B). Those changes were not found under gait with eyes open.
Discussion
Visual information along with head stability during gait are crucial for smooth gait motor performance, in addition to normal peripheral vestibular function. Head stability during dynamic locomotor performance is affected by peripheral vestibular lesion. Unilateral labyrinthectomy is well known to cause head nystagmus, head deviation, head tilt and shifted locomotion. However, how unilateral partial or total dysfunction of the Otolith end organs can affect locomotor performance is not well documented, especially in terms of head stability during gait. Our recent study showed greater head movement during locomotion in patients with AN [7]. This phenomenon could be attributable to some dysfunction due to the tumor. Recently it has become possible to examine Otolith organ functions separately, using oVEMP for the utricle and cVEMP for the saccule [6]. The main objective of this study was thus to examine the relationship between Otolith organ dysfunction and head stability during gait. We evaluated Otolith function using oVEMP and cVEMP tests, and found that most AN cases displayed saccular or utricular dysfunction, while a smaller proportion (3 cases) showed normal responses despite the tumor. Those are the cases who had intra-canalicular tumors. We sometimes encounter this kind of discrepancy between tumor presence and functional status. The results of this study showed no significant change between AN cases with normal VEMPs and controls. However, cases with VEMP abnormality showed a unique instability during gait.
Saccular neurons have been shown to have strong projections to the neck muscles and weak projections to the oculomotor system, while utricular afferents have strong projections to eye muscles [8]. Measuring oculomotor responses (i.e., oVEMP) thus predominantly evaluates utricular function, whereas measuring neck muscle responses (i.e., cVEMP) predominantly involves saccular function [9]. As a result, oVEMP is believed to predominantly test contralateral utricular function, while cVEMP predominantly tests ipsilateral saccular function [10-11].
Research from healthy subjects also suggests that vestibular information contributes to head stabilization during dynamic tasks to enable successful gaze control [12].
Pozzo et al. [13] have suggested that the head acts as a frame of reference during dynamic tasks because both the vestibular and visual systems are able to relay information regarding the head in space. To ensure that information from these sensory systems is appropriate, the head has been found to be stabilized in space in the pitch [13-15], yaw [16] and roll planes [17], including evidence of anticipatory control of head acceleration in the pitch plane during forward locomotion [18].
Our previous study found that head movement in the pitch and roll planes, during the eyes-closed condition, was significantly greater in AN patients than in controls, indicating that visual cues are important for head stability during walking and that asymmetric Otolith function caused by tumor could play some role in head stability [19]. The present study found that horizontal head movement during walking with eyes open or closed was significantly greater in AN patients with utricular dysfunction when compared with those in the control group. However, no significant difference in up-down head movement was identified between patients and the control group (up-down head movement was stable regardless of Otolith organ dysfunction). We also found that head movement in the pitch plane during walking with eyes open and closed, and in the roll plane with eyes closed was significantly greater in AN patient with saccular dysfunction when compared with those in the control group. It is deemed that greater head movement in yaw plane under eyes open in AN cases with abnormal cVEMP test and normal oVEMP test results could not be significant, since this change in head movement was not confirmed under eyes closed just like all other significant changes in other planes.
Changes in head stability during gait might be neither complained of nor apparent on visual observation. However, we have to note that even this kind of insidious Otolith dysfunction can affect head stability during locomotion, especially under deprivation of visual cues, just like the appearance of gait-phase instability even in cases with small AN [20].
References
- Komatsuzaki A, Tsunoda A. Nerve origin of the acoustic neuroma. J Laryngol Otol. 2001; 115: 376-379.
- Minor LB. Gentamicin-induced bilateral vestibular hypofunction. JAMA. 1998; 279: 541-544.
- De Burlet HM. Zur Innervation der Macula sacculi bei Säugetieren. Anat Anzeiger. 1924; 58: 26-32.
- De Burlet H. Zur vergleichenden Anatomie der Labyrinthinnervation. J Comp Neurol. 1929; 47:155-169.
- Ishikawa K, Wang Y, Wong WH, Shibata Y, Itasaka Y. Gait instability in patients with acoustic neuroma. Acta Otolaryngol. 2004; 124: 486-489.
- Curthoys IS. The interpretation of clinical tests of peripheral vestibular function. Laryngoscope. 2012; 122: 1342-1352.
- Itasaka Y, Ishikawa K, Omi E, Koizumi K. Three-dimensional motion analysis of head movements during locomotion in patients with acoustic neuromas- focused on the relationship with peripheral vestibular function. Equilibrium Res. 2015; 74: 20-28.
- Uchino Y, Sasaki M, Sato H, Bai R, Kawamoto E. Otolith and canal integration on single vestibular neurons in cats. Exp Brain Res. 2005; 164: 271-285.
- Curthoys IS, Vulovic V, Burgess AM, Cornell ED, Mezey LE, Macdougall HG, et al. The basis for using bone-conducted vibration or air-conducted sound to test otolithic function. Ann N Y Acad Sci. 2011; 1233: 231-241.
- Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol. 2010; 121: 132-144.
- Curthoys IS. A balanced view of the evidence leads to sound conclusions. A reply to J.G. Colebatch "Sound conclusions?". Clin Neurophysiol. 2010; 121: 977-978.
- Pozzo T, Berthoz A, Lefort L. Head stabilization during various locomotor tasks in humans: normal subjects. Exp Brain Res 1990; 82: 97-106.
- Lang J, Ishikawa K, Hatakeyama K, Wong WH, Yin M, Saito T. 3D body segment oscillation and gait analysis for vestibular disorders. Auris Nasus Larynx. 2013; 40: 18-24.
- Pozzo T, Bethoz A, Lefort L, Vitte E. Head stabilization during various locomotor tasks in humans. II. Patients with bilateral vestibular deficits. Exp Brain Res. 1991; 85: 208-217.
- Shupert CL, Horak FB. Effects of vestibular loss on head stabilization in response to head and body perturbations. J Vestib Res. 1996; 6: 423-437.
- Cromwell RL, Newton RA, Carlton LG. Horizontal plane head stabilization during locomotor tasks. J Mot Behav. 2001; 33: 49-58.
- Pozzo T, Levik Y, Berthoz A. Head and trunk movements in the frontal plane during complex dynamic equilibrium tasks in humans. Exp Brain Res. 1995; 106: 327-338.
- Prince F, Winter DA, Stergiou P, Walt SE. Anticipatory control of upper body balance during human locomotion. Gait Posture. 1994; 2: 19-25.
- Aboshanif M, Ishikawa K, Itasaka Y, Omi E, Koizumi K, Suzuki S, et al. Changes in gait detected by three-dimensional motion analysis in patients with acoustic neuroma. J Otolaryngol ENT Res. 2015; 2: 00032.
- Yin M, Ishikawa K, Omi E, Saito T, Itasaka Y, Angunsuri N. Small vestibular schwannomas can cause gait instability. Gait Posture. 2011; 34: 25-28.