
Research Article
Austin J Otolaryngol. 2016; 3(1): 1072.
Nasal Fungal Pathology and Trichothecenes Associated with Water-Damaged School and Home
Dennis DP¹ and Thrasher JD²*
¹Atlanta Center for ENT & Facial Plastic Surgery, Atlanta, Georgia, USA
²Board of Directors- Global Indoor Health Network and National Toxic Encephalopathy-foundation, Citrus Heights, CA, Las Vegas, NV, USA
*Corresponding author: Thrasher JD, Board of Directors and Research Committee, Global Indoor Health Network, Henderson, Nevada
Technical Director, National Toxic Encephalopathy Foundation, Las Vegas, Nevada
Holistic Approach to Optional Health and Integrative Treatment of Complex Diseases, Tarrytown, NY, Progressive Health Care, Benson, Arizona
Atlanta Center for ENT & Facial Plastic Surgery, Atlanta, Georgia, USA
Received: May 11, 2016; Accepted: May 31, 2016; Published: June 02, 2016
Abstract
A 52 year old immunocompetent woman exposed to fungi in a waterdamaged classroom and possibly in her home was evaluated for rhinosinusitis. CT scan of the sinuses revealed a nodular mass in the left ethmoid. Swab of the nasal mucosa cultured on SDA agar plate identified bacteria (TNTC), Candida (TNTC) and at least 10 other genera of fungi. A brown halo developed around the nasal mucosa on the SDA agar. The halo was sampled and revealed the presence of trichothecenes at 0.28 ppb. The surgical removal, fungal IgG antibodies and treatment of the fungal nodule, culture and identification of the trichothecenes are described in full in this communication.
Keywords: Fungus; Rhinosinusitis; Hypersensitivity; Aspergillus; Ethmoid sinus mucosa
Introduction
Chronic Fungal Rhinosinusitis (CRS) is relatively common, but often it is a misdiagnosed disease process of the nasal mucosa and paranasal sinuses [1,2]. It has been suggested that eosinophilic major basic protein may be involved in the inflammatory response of CRS [2,3]. However, the main diagnostic approach to identify fungal rhinosinusitis and CRS is an allergic condition related to Type I (IgE) hypersensitivity [4,5]. IgE fungal hypersensitivity occurs in 30 % of CRS patients, but elevated IgG fungal antibodies are present in about 90 % of CRS cases [1]. The incidence of the disease is 37 million cases that encompass a wide range of pathological and immune responses involving the innate immune system (Th-2 chemokines and cytokines), dysfunction of the nasal epithelial immune response and actual mucosal invasion by bacteria and fungi [6-10]. Pathological responses include invasive, chronic granulomatous and allergic conditions. A recent attempt was made to classify the various types of fungal sinusitis [11]. The current schema still includes 1) invasive diseases (acute invasive, granulomatous invasive and chronic) FRS and 2) noninvasive disease (saprophytic fungal infections, fungal ball and fungus related eosinophilic FRS that includes AFRS (allergic fungal rhinosinusitis). Thus, FRS results from multiple fungal genera, including Aspergillus species [12-16]. Aspergillus species are involved in invasive CRS in immunocompetent individuals [17-20]. In all cases the condition is refractory to antibiotic regimens and is improved with intranasal antifungals [2,19,20]. The use of corticosteroids should be limited because of the potential for suppression of the neutrophil migration and killing action of fungal spores and hyphae by both neutrophils and macrophages [21-23]. In addition, Aspergillus sinusitis can mimic malignant disease and even appear as pituitary tumor and a neuroblastoma [24-27]. Mimicry of a variety of disease conditions is common to individuals who have developed Sarcoidosis [28, 29]. This is raised because recent reports have identified Sarcoidosis in fungal exposed patients [30-32]. Presented herein is a case of a 52 year old immunocompetent woman who, following exposure to the bio-contaminants present in her waterdamaged classroom and house, developed bacterial, Candida and several genera of fungi in a nasal mucosa and ethmoid sinus infection. Trichothecene mycotoxins in the nasal mucosa were symptomatic, not responding to medical therapy and required endoscopic sinus surgery to improve symptoms.
Materials and Methods
Patient history
The patient is a 52 year old woman seen on 07/29/14. She had chronic nasal and sinus congestion consistent with chronic rhinosinusitis and sought diagnostics and treatment. She had no history of chronic sinusitis, use of antibiotics but had positive sinus pressure in her ear and head and post-nasal drip. Her home and school had water intrusion. Environmental tests revealed Stachybotrys and Penicillium/Aspergillus spores in the indoor air school. She had shortness of breath that was improved by Itraconozole provided by another physician.
Mold assessment
The inspection and assessment for fungi and water damage in the school classrooms was done by Quality Environmental Solutions and Technologies, Inc., Wappinger Falls, NY according to the guidelines of the New York City Department of Health.
Nasal surgery and procedures
Endoscopic Sinus Surgery was done under general anesthesia, the ethmoid sinuses were entered, and the mucosa was a yellowish brown with some areas of normal color. All affected mucosa was removed and some specimens were sent to Peachtree Laboratory & Associates, Atlanta GA that resulted in the diagnosis of Chronic Sinusitis (left and right ethmoid, and frontal). No hyphae, eosinophils, or mucosal invasion was seen on microscopic exam. Some of the mucosa was placed in a SDA agar plate to grow out mold, but no mold grew, a brown halo developed around the sinus mucosal tissue on day 4 and it was sent to Real Time Laboratories for mycotoxins testing of the brown substance in the agar. At conclusion of the procedure, all paranasal sinuses were irrigated with Amphotericin-B solution of 50mg in 500ml sterile water. And following patient nebulizer Amphotericin-B at 3mg per 30ml of sterile water in through nose and out through mouth for 6 weeks; Itraconozole 200mg 2x day for 2 months. Nystatin 1, 2x day for Candida control; Hydrocortisone 5mg 2x day for adrenal insufficiency, Thyroid 50mg AM, Levothyroxine 50mg pm.
Additional medications from other physicians included: Chorella 8 tabs 2x day, Grape Seed extract 500mg po bid (unknown amount) Cholestyramine 4gm po bid, monolaurin 600mg po bid means 1 2x day; Intramax liquid vitamin, 1 oz daily (Drucker labs); Transfer factor sublingual spray for antifungal & bacterial immune support 5 sprays sublingual 2x day and 1-3 Beta Glucan (Microbalance Health Products); Methyl B12, Folic acid, Probiotic (VSL#3); and Xymogen I5 to detoxify phase I and II of liver detoxification pathways (Xymogen.com). None of these supplements were evaluated for this communication.
CT scan
The CT scan was performed at MRI and Imaging at Midtown, Atlanta, Georgia on 8-8-14 using coronal view at 1mm cuts resolution.
SDA agar plates
Sabouraud Dextrose agar plates were purchased from BD product center, San Diego, CA. A sterile cotton colgi swab was taken from the nasal cavity. The SDA plate was then inoculated, sent to Immunolytics, Albuquerque, NM, and cultured for 5 days in the dark at room temperature. The colonies of fungi were identified by Immunolytics.
IgG fungal antibodies
The serum from the patient was sent to Commonwealth Medical laboratories, Inc., Warrenton VA. IgG antibodies against 11 fungi and Brewer’s yeast was ordered. The results were expressed as μg/ml.
Mycotoxin identification
The SDA agar plate and urine specimen were sent by overnight carrier to Real Time Laboratories, Carrollton, TX. The urine and the brown halo on the SDA agar around the ethmoid mucosa (Figure 1) were tested for Aflatoxin, Ochratoxin A and Trichothecenes as previously published [33].
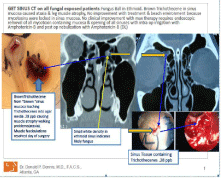
Figure 1: This figure summarizes the pathology and CT scan of the fungal
ball in the ethmoid sinus as follows:
(1) On the left side the blue arrows point the dark brown ethmoid sinus mucosa
and the brown halo around the ethmoid tissue in the SDA agar is mycotoxins
leaching out of the ethmoid tissue, was identified as Trichothecenes at
0.28 ppb. (2) In the center the yellow arrows point to the ethmoid fungal
ball. (3) The blue arrows are pointing at the sinus tissue that contained the
trichothecenes note in (1) above.
Sinus mucosa pathology
Sinus mucosa was sent to pathology at Peachtree Laboratory Associates, Atlanta, GA. Left and right ethmoid sinus and right frontal sinus mucosa was submitted for GMS stain for microscopic exam for hyphae and eosinophil identification and histological examination.
Results
Inspection and assessment
The inspection and assessment was limited to visual observations for water intrusion and mold growth in the HVAC ducts, total airborne spores (Three Aero-Spore-Trap cassettes) and one tape lift sample. Water intrusion was noted in several places with the presence of suspected microbial growth. Sampling was not done on the suspected microbial growth. The HVAC system was visually inspected, but sampling of dust was not done. Airborne spores included Ascospores, Basidiospores, Chaetomium, Aspergillus/ Penicillium spores, Curvularia, Rhizopus and Stachybotrys. One air sample had Stachybotrys at 66.2 % of the total of 7,100 spores per cubic meter. No attempt was made to identify species of fungi. However, Chaetomium and Stachybotrys are hydrophilic fungi and require =90 % water content. Thus, based upon the visual observation revealing water intrusion and the detection of Chaetomium and Stachybotrys, the school rooms had experienced serious intrusion events.
Patient evaluation
Physical examination revealed muscle wasting in her hands and legs, ataxia from weakness of the legs. She stated that she did not feel better at the beach. She had a low energy level of 4 with 10 being normal. She experienced an allergic complex to foods, stomach pressure, leaky gut syndrome, gluten sensitivity, muscle and joint pain, weakness, memory loss, concentration problems, blurred vision, numbness and tingling, anxiety, depression, irritability, and urticaria. She did not experience improvement with maximal medical therapy, including moving out of the house and not taking any household belongings with her.
Results of CT scan and Figure 1
CT scan revealed a right ethmoid fungus appearing mass with mucosal thickening of the left posterior ethmoid sinus, thickening in the right frontal and ethmoid sinuses (Figure 1). The infundibula were narrowed and the left frontal sinuses appeared clear. A deviated nasal septum to the right with turbinate hypertrophy was present.
SDA Culture results
The results of the culture of the ethmoid specimen are shown in (Figure 2) and summarized in (Table 1). The bacteria were not identified because it was a fungal identification culture. However, colonies of fungal genera were identified. The number of colonies ranged from 1 to TNTC as follows: Candida (TNTC); Fusarium (3); Penicillium (3); Helminthosporium (2); Aspergillus (2); Alternaria (2); and other fungi at (1).
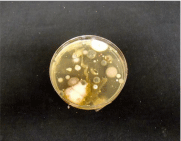
Figure 2: This figure shows the SDA agar plate demonstrating the fungal
colonies that appeared following culturing of the nasal swab sample for
5 days in the dark at room temperature. The number of colonies of each
fungus ranged from as low as one (Microsporium) to nine (Rhodotorula). The
bacteria and Candidia were TNTC.
Cultured Organism
Number of Colonies
Bacteria
TNTC
Candida
TNTC
Cladosporium
5
Penicillium
2
Epicoccum
2
Microsporium
1
Rhodotorula
9
Aspergillus
2
Table 1: Bacteria and Fungi cultured from the nasal mucosa of the ethmoid sinus. The number of colonies of each fungal colony observed on the SDA agar plate ranged from 1 to TNTC, while the bacteria were too numerous to count.
IgG antibodies in serum
The IgG serum antibodies detected by Commonwealth Laboratories showed an immune response to the eleven species of fungi is summarized in (Table 2). IgG antibodies were directed against all species of fungi with the exception of Cladosporium herbarum and Brewer’s yeast.
Test
Class
Concentration (”g/ml)
Yeast Brewer’s
Neg
0.46
Penicillium. notatum/chrysogenum
III
12.37
Cladosporium herbarum
Neg
0.81
Aspergillus Fumigatus
II
7.14
Mucor racemous
I
1.08
Candida albicans
IV
52.01
Alternaria. ternius (alternata)
II
4.43
Helminthosporium halodes
II
4.97
Curvularia lunata
I
2.10
Fusarium oxsporum
III
24.92
Epicoccum nigrum
I
2.18
Acremonium killense (cephalsosp)
I
1.22
Table 2: This table summarizes the IgG antibodies to fungi detected in the serum of the patient with a comment regarding interpretation of the degree of sensitivity as recommended by the Diagnostic Laboratory.
Mycotoxins in urine and SDA plate
The results of the mycotoxins present in the urine sample and the halo area around the ethmoid sinus mucosa in (Figure 1) are summarized in (Table 3). Trichothecenes were detected at 0.52 ppb with a limit of detection of 0.2 ppb. Ochratoxin A was detected at 1.77 ppb, which is below the detection limit of 2 ppb. However, the patient had received prior treatment with Sporanox, which can result in decreased concentration of urine mycotoxins. The halo around the ethmoid sinus mucosa on the SDA agar plate in (Figure 1) contained trichothecenes at 0.28 ppb.
Sample
Aflatoxin
Ochratoxin A
Trichothecene
Urine
N.D.
1.77
0.52
Brown Halo – SDA Plate
N.D.
N.D.
0.28
N.D. = Not Detected
Aflatoxin Limit of Detection = 1.0 ppb
Ochratoxin A Limit of Detection = 2.0 ppb
Trichothecene Limit of Detection = 0.2 ppb
Table 3: This Table presents the concentrations in ppb of mycotoxins in the urine sample from the patient and trichothecene detected in fungal colony in Figure 1.
Pathology
Histological examination: Left and right ethmoid sinus mucosa, and right frontal sinus mucosa was submitted. GMS stain microscopic exam did not show fungi. Histology showed chronic sinusitis in all specimens. No eosinophils were seen. The most likely Fungal Chronic Rhinosinusitis (FCRS) classification was AFRS because it was non invasive on histology exam. Fungus was cultured from the nose prior to treatment and the Sinus CT scan shows a small polyp density usually associated with eosinophils. Although after treatment with antifungals orally and via nebulization with steroids, as expected, no fungi or eosinophils were seen in the surgical specimen.
Results of surgery and prescribed medications: Prior to surgery she had leg muscle atrophy, weakness, ataxia, and leg fasciculations nightly while sleeping for approximately 10 years. After surgery she experienced resolution of the leg fasciculations during sleep and the weakness and ataxia improved.
The medical protocol improved her symptoms very slightly by getting her energy level from a 4 to a 5 with normal of 10. Improvement was minimal. Medical protocol was: Chorella 8 tabs 2x day, Grape Seed extract 500mg po bid, Cholestyramine 4gm po bid, monolaurin 600mg po bid means 1 2x day; Intramax liquid vitamin, 1 oz daily (Drucker labs); transfer factor sublingual spray for antifungal & bacterial immune support 5 sprays sublingual 2x day and 1-3 Beta Glucan (Microbalance Health Products); Methyl B12, Folic acid, Probiotic (VSL#3); and Xymogen I5 to detoxify phase I and II of liver detoxification pathways (Xymogen.com); Itraconozole 100-200 mg bid, Amphotericin-B nasal nebulization bid, saline nose irrigation bid. None of these supplements were evaluated for this communication.
Discussion
The patient in this case study was exposed to the microbial conditions that existed in her classroom and home. As a result she did develop health conditions that made her seek medical attention. An initial trial of Sporanox prescribed by another physician lessened her shortness of breath. However, her conditions of fatigue, muscle fasciculations and congestion in her head and nasal cavity resulted in her consult. The SDA culture from a nasal swab resulted in identifying bacteria at TNTC and Candida (TNTC) as well as several genera of fungi (Figure 2 and Table 1). In addition, IgG antibodies were positive for 10 species of fungi present in water-damaged indoor environments. The CT scan revealed a dense nodule in her left ethmoid sinus. Surgery was undertaken to remove the foreign nodule. At surgery the sinus mucosa representing the ethmoid nodule was placed on the SDA agar plate. Following 5 days of incubation a brown halo developed around the ethmoid tissue. The halo on the SDA plate tested positive for trichothecenes at 0.28 ppb that apparently leached from the mucosa. In addition her urine contained trichothecenes at 0.52 ppb and ochratoxin at 1.77 pbb (Figure 1, Table 3). She was then placed on intranasal amphotericin-B nebulization, which killed the mucosal fungus. The surprise of this procedure was the detection of leached trichothecenes in the absence of fungal growth on the SDA plate. The absence of fungal growth may have resulted from a short culture time and lack of optimum temperatures of 35-37 °C for Aspergillus species (55, 56). Although the detection of trichothecene in the brown halo was an accidental discovery, the discovery should alert other ENT physicians to look for the presence of mycotoxin in affected nasal mucosa.
The role of fungi in chronic rhinosinusitis was initially introduced by Ponikau et al. [1]. Since then, numerous studies have been published demonstrating the role of fungi in Type I IgE Hypersensitivity, chronic inflammation, innate immunity and invasion of the orbit and central nervous system [1-27]. The invasion of surrounding tissues occurs in immunocompetent individuals [17,19,20,26,34,35]. Recent publications have presented evidence that fungi are present in brain regions of Alzheimer’s patients and in the cerebrospinal fluid and brain tissue from patients with amyotrophic lateral sclerosis [36,37]. We suggest that the classification of invasive fungal infections outlined by Chakrabarti et al. be seriously considered with respect to patients with fungal sinusitis [11]. Individuals exposed to fungi and bacteria in water-damaged buildings develop multiple symptoms and upper and lower respiratory infections, sarcoidosis, asthma and asthmalike symptoms [38-45]. Thus, it appears that fungi and bacteria along with their secondary metabolites are important factors in illness of occupants in water- damaged homes, schools and office buildings. Recent case reports on families and office employee are supportive of this conclusion [26,30-32, 43-45].
The patient in this case presentation was exposed to fungi in a water-damaged classroom. The environmental investigation revealed elevated levels of ascospores, Basidiospores and spores of Aspergillus, Penicillium and Stachybotrys. Fungal fragments less than one micron containing trichothecene mycotoxins have been identified in the indoor air of water-damaged buildings [46,47]. The fungal fragments are aerosolized from mold colonies by air current simulating the HVAC system [48,49]. The aerodynamic characteristics and respiratory deposition of these fungal fragments occur in the nasal cavity and lungs [50]. In addition, mycotoxins are readily deposited in HVAC systems [51-53]. Thus, it is not surprising occupants of water-damaged buildings and homes have trichothecene mycotoxins in their sera, nasal cavity, tissues, and urine [19,20,33,43-45,54].
Conclusion
This case report illustrates that since most airborne mycotoxins enter through the nose, some remain in concentrations high enough to cause systemic symptoms and therefore must be removed via Endoscopic Sinus Surgery (ESS) to relieve symptoms in those who fail environmental and medical therapy. In this case accompanied with symptoms of leg muscle atrophy, ataxia, muscle weakness, and muscle fasciculation, the symptoms improved after endoscopic sinus mucosal removal and irrigation with Amphotericin-B. In addition, the presence of Candida at TNTC in the nasal cavity may have resulted from the immunosuppressive effects of mycotoxins, including trichothecenes. This may also account for the overgrowth of bacteria detected on the SDA plate. Although the detection of trichothecene in the brown halo was an accidental discovery, the discovery should alert ENT physicians to the presence of mycotoxin in affected nasal mucosa. Sinus surgery should be considered for patients who fail medical and environmental treatment.
References
- Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E, Gaffey TA. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999; 74: 877-884.
- Ponikau JU, Sherris DA, Kephart GM, Kern EB, Congdon DJ, Adolphson CR. Striking deposition of toxic eosinophil major basic protein in mucus: implications for chronic rhinosinusitis. J Allergy Clin Immunol. 2005; 116: 362-369.
- Ponikau JU, Winter LA, Kephart GM, Squillace DL, Hershcovitch MD, Moon S. An immunologic test for chronic rhinosinusitis based on free intranasal eosinophilic major basic protein. Int Forum Allergy Rhinol. 2015; 5: 28-35.
- Bent JP, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994; 111: 580-588.
- Glass D, Amedee RG. Allergic fungal rhinosinusitis: a review. Ochsner J. 2011; 11: 271-275.
- Porter PC, Lim DJ, Maskatia ZK, Mak G, Tsai CL, Citardi MJ. Airway surface mycosis in chronic TH2-associated airway disease. J Allergy Clin Immunol. 2014; 134: 325-331.
- Kohanski MA, Lane AP. Sinonasal epithelial cell response to Staphylococcus aureus burden in chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2015; 141: 341-349.
- Ramanathan M Jr, Lee WK, Spannhake EW, Lane AP. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. in Am J Rhinol. 2008; 22: 115-121.
- Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010; 18: 21-26.
- Schleimer RP, Kato A, Peters A, Conley D, Kim J, Liu MC. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009; 6: 288-294.
- Chakrabarti A, Denning DW, Ferguson BJ, Ponikau J, Buzina W, Kita H. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope. 2009; 119: 1809-1818.
- Katzenstein AL, Sale SR, Greenberger PA. Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy Clin Immunol. 1983; 72: 89-93.
- Schubert MS. Allergic fungal sinusitis: pathophysiology, diagnosis and management. Med Mycol. 2009; 47: 324-330.
- Shin SH, Ponikau JU, Sherris DA, Congdon D, Frigas E, Homburger HA. Chronic rhinosinusitis: an enhanced immune response to ubiquitous airborne fungi. J Allergy Clin Immunol. 2004; 114: 1369-1375.
- Reddy CE, Gupta AK, Singh P, Mann SB. Imaging of granulomatous and chronic invasive fungal sinusitis: comparison with allergic fungal sinusitis. Otolaryngol Head Neck Surg. 2010; 143: 294-300.
- Milroy CM, Blanshard JD, Lucas S, Michaels L. Aspergillosis of the nose and paranasal sinuses. J Clin Pathol. 1989; 42: 123-127.
- Siddiqui AA, Shah AA, Bashir SH. Craniocerebral aspergillosis of sinonasal origin in immunocompetent patients: clinical spectrum and outcome in 25 cases. Neurosurgery. 2004; 55: 602-611.
- Sivak-Callcott JA, Livesley N, Nugent RA, Rasmussen SL, Saeed P, Rootman J. Localised invasive sino-orbital aspergillosis: characteristic features. Br J Ophthalmol. 2004; 88: 681-687.
- Dennis DP. Chronic sinusitis: defective T-cells responding to superantigens, treated by reduction of fungi in the nose and air. Arch Environ Health. 2003; 58: 433-441.
- Dennis DP, Robertson D, Curtis L, Black J. Fungal exposure endocrinopathy in sinusitis with growth hormone deficiency: Dennis-Robertson syndrome. Toxicol Indust Health. 2009; 25: 669-680.
- Gan WQ, Man SF, Sin DD. Effects of inhaled corticosteroids on sputum cell counts in stable chronic obstructive pulmonary disease: a systematic review and a meta-analysis. BMC Pulm Med. 2005; 5: 3.
- Palmer LB, Greenberg HE, Schiff MJ. Corticosteroid treatment as a risk factor for invasive aspergillosis in patients with lung disease. Thorax. 1991; 46: 15-20.
- Philippe B, Ibrahim-Granet O, Prévost MC, Gougerot-Pocidalo MA, Sanchez Perez M, Van der Meeren A. Killing of Aspergillus fumigatus by alveolar macrophages is mediated by reactive oxidant intermediates. Infect Immun. 2003; 71: 3034-3042.
- Larraaga J, Fandio J, Gomez-Bueno J, Rodriguez D, Gonzalez-Carrero J, Botana C. Aspergillosis of the sphenoid sinus simulating a pituitary tumor. Neuroradiology. 1989; 31: 362-363.
- Lee JH, Park YS, Kim KM, Kim KJ, Ahn CH, Lee SY. Pituitary aspergillosis mimicking pituitary tumor. AJR Am J Roentgenol. 2000; 175: 1570-1572.
- Gray MR, Thrasher JD, Hooper D, Dumanov MJ, Cravens R, Jones T. Sphenoid aspergilloma: Diagnoses as a malignancy: A case report. Otolaryngol. 2015; 5: 3.
- Daghistani KJ, Jamal TS, Zaher S, Nassif OI. Allergic aspergillus sinusitis with proptosis. J Laryngol Otol. 1992; 106: 799-803.
- Gerke AK. Morbidity and mortality in sarcoidosis. Curr Opin Pulm Med. 2014; 20: 472-478.
- Rao DA1, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013; 39: 277-297.
- TerÄelj M, Salobir B, Zupancic M, Wraber B, Rylander R. Inflammatory markers and pulmonary granuloma infiltration in sarcoidosis. Respirology. 2014; 19: 225-230.
- Tercelj M, Salobir B, Harlander M, Rylander R. Fungal exposure in homes of patients with Sarcoidosis – an environmental exposure study. Environ Health. 2011; 20: 8.
- Tercelj M, Salobir B, Zupancic M, Rylander R. Sarcoidosis treatment with antifungal medication: A Follow-up. Pulmonary Med. 2014; 4.
- Hooper DG, Bolton E, Guilford FT. Straus DC. Mycotoxin detection in human samples from patients exposed to environmental molds. Int J Mol Sci. 2009; 10: 1465-1475.
- Aggarwal E, Mulay K, Menon V, Sundar G, Honavar SG, Sharma M. Isolated orbital aspergillosis in immunocompetent patients: A multicenter study. Am J Ophthalmol. 2016; 165: 125-132.
- Srinivasan US. Intracranial aspergilloma in immunocompetent patients successfully treated with radical surgical intervention and antifungal therapy: case series. Ann Acad Med Singapore. 2008; 37: 783-787.
- Alonso R, Pisa D, Marina AI, Morato E, Alberto Rábano, Izaskun Rodal. et al. Evidence for fungal infection in cerebrospinal fluid and brain tissue from patients with amyotrophic lateral sclerosis. Inter J Biol Sci. 2015; 11: 546-558.
- Pisa D, Alonso R, Rábano A, Rodal I, Carrasco L. Different Brain Regions are Infected with Fungi in Alzheimer's Disease. Sci Rep. 2015; 5: 15015.
- Gray MR, Thrasher JD, Crago R, Madison RA, Arnold L, Campbell AW. Mixed mold mycotoxicosis: immunological changes in humans following exposure in water-damaged buildings. Arch Environ Health. 2003; 58: 410-420.
- Curtis L, Lieberman A, Stark M, Rea W, Vetter M. Adverse health effects of indoor moulds. J Austral College Nutr Environ Med. 2004; 23: 3-8.
- Fisk WJ, Eliseeva EA, Mendell MJ. Association of residential dampness and mold with respiratory tract infections and bronchitis: a meta-analysis. Environ Health. 2010; 9: 72.
- Park JH, Cox-Ganser J, Rao C, Kreiss K. Fungal and endotoxin measurements in dust associated with respiratory symptoms in a water-damaged office building. Indoor Air. 2006; 16: 192-203.
- Laney AS, Cragin LA, Blevins LZ, Sumner AD, Cox-Ganser JM, Kreiss K, et. al. Sarcoidosis, asthma, and asthma-like symptoms among occupants of a historically water-damaged building. Indoor Air. 2009; 19: 83-90.
- Thrasher JD, Gray MR, Kilburn KH, Dennis DP, Yu A. A water-damaged home and health of occupants: a case study. J Environ Public Health. 2012; 312836.
- Thrasher JD, Hooper D, Taber J. Family of six, their health, and the death of a 16 month old male from pulmonary hemorrhage: Identification of mycotoxins and mold in the home and lungs, liver and brain of deceased infant. Internat J Clin Toxicol. 2014; 2: 1-10.
- Thrasher JD, Prokop C, Roberts C, Hooper D. A family with ME/CFS following exposure to molds, mycotoxins and bacteria in a water-damaged home: A case report. Internat J Clin Toxicol 4 (in press). 2016.
- Brasel TL, Douglas DR, Wilson SC, Straus DC. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene in particulates smaller than conidia. Appl Environ Microbiol. 2005; 71: 114-122.
- Gottschalk C, Bauer J, Meyer K. Detection of satratoxin g and h in indoor air from a water-damaged building. Mycopathologia. 2008; 166: 103-107.
- Mensah-Attipoe J, Saari S, Veijalainen A-M, Pasanen P, et al. Sci Total Environ.
- Garny RL, Ćawniczek-WaĆczyk A. Effect of two aerosolization methods on the release of fungal propagules from a contaminated agar surface. Ann Agric Environ Med. 2012; 19: 279-284.
- Seung-Hyun Choa, Sung-Chul Seoa, Detlef Schmechelb, Sergey A. Grinshpuna, Tiina Reponena. Aerodynamic characteristic and respiratory deposition of fungal fragments. Atmos Environ. 2005; 39: 5454-5465.
- Smoragiewicz W, Cossette B, Boutard A, Krzystyniak K. Trichothecene mycotoxins in the dust of ventilation systems in office buildings. Int Arch Occup Environ Health. 1993; 65: 113-117.
- Hintikka F, Holopainen R, Asola A, Jestoi M, Peitzsch M, Kalso S. et al. Mycotoxin in the ventilation systems of four schools in Finland. World Mycotoxin J. 2009; 2: 369-379.
- Thrasher JD. Mycotoxins in dust samples from HVAC ducts and refrigerator compressors in water-damaged homes (Manuscript in preparation). 2016.
- Brasel TL, Campbell AW, Demers RE, Ferguson BS, Fink J, Vojdani A. Detection of trichothecene mycotoxins in sera from individuals exposed to Stachybotrys chartarum in indoor environments. Arch Environ Health. 2004; 59: 317-323.
- McClenny N. Laboratory detection and identification of Aspergillus species by microscopic observation and culture: the traditional approach. Medical Mycology. 2005; 43.
- Tarrand JJ, Han SY, Kontoyiannis DP, May GS. Aspergillus hyphae in infected tissue: Evidence of physiologic adaptation and effect on culture recovery. J Clinical Microbiol. 2005; 43: 382-386.