
Research Article
Austin J Otolaryngol. 2016; 3(2): 1074.
Distraction Osteogenesis in Pierre Robin Sequence
Faria R¹* and Valladares S²
¹Maxillofacial Surgeon Hospital Del Salvador, Maxillofacial Surgeon Hospital San Borja Arriarán, Member of Chilean Society of Maxillofacial Surgery, Master in Education, Professor of Oral and Maxillofacial Surgery University of Chile, Santiago, Chile
²Oral and Maxillofacial Surgery, Hospital El Carmen de Maipú, Oral surgery instructor, Faculty of Medicine Pontifical Catholic University of Chile. Santiago, Chile
*Corresponding author: Faria R, Department of Maxillofacial Surgery, Universidad de Chile, Santiago, Chile
Received: May 10, 2016; Accepted: July 01, 2016; Published: July 04, 2016
Abstract
The Pierre Robin sequence is a pathology derived from an alteration on the first and second branquial arch. The major problems of these patients are: the breathing problems due to micrognathia and glossoptosis that causes an upper airway obstruction.
The management has changed in the last years, with new surgical techniques to allow treating etiologic problems.
We did a review of literature about different kinds of treatment and show a 25 patient group treated with Pierre Robin Sequence.
Patients and Methods: We treated 25 new born patients, with Pierre robin sequence and severe breathing problems. 6 of them need mechanical ventilator assistance. We made an average of 18,8mm of mandibular distraction to improve their breathing parameters.
Results: After the first 6mm of mandibular distraction, spontaneous ventilation was observed, in the majority of these patients. At the end of distraction, the breathing function and facial balance was obtained.
Conclusion: Mandibular distraction osteogenesis is the best etiological surgical treatment for the Pierre Robin sequence with respiratory obstruction.
Keywords: Distraction osteogenesis; Pierre Robin sequence; Micrognathia; Upper airway obstruction
Introduction
The Pierre Robin sequence is one of much pathology caused by developmental alterations of the 1st and 2nd branchial arch. It has been suggested to be an autonomic recessive disorder, characterized by mandibular hypoplasia, resulting in a cleft palate and Glossoptosis [1]. A Cleft palate makes sucking and swallowing difficult, allowing easy access of fluids into the larynx and Glossoptosis contributes to respiratory alteration. The mandible`s poor development offers an inadequate space for the tongue to descend, a development which usually takes place during the 7th week of in-uterus life [2]. The vertical position of the tongue is the main factor that obstructs the horizontalization of the palate process, causing a clef palate.
The physiological alterations derived from this sequence are various. The most serious alteration and complex is the compromise of the Upper Airway (U.A.). The U.A. `s obstruction can present several degrees of severity, from a simple chronic airway limitation and episodic apneas, to severely obstruction the ventilation causing respiratory acute insufficiencies.
Along with the respiratory problems, swallowing alterations can make oral nutrition difficult, which frequently leads to malnutrition. The gastroesophagic reflux, secondary to the chronic limitation of the U.A. also contributes to the nutritional problems, often making necessary the installation of naso-gastric probe or even a gastrectomy [3].
The aim of initial management of these new born is to correct ventilation, which can be achieved in a decubital - prono position. However a great vital risk remains with these conservative maneuvers.
Duhamel [4] reports a 50% mortality rate, while Bush [5] and Dykes [6] mention a rate between 10% to 30%.
When these maneuvers are insufficient, a therapy of oxygen administration through masks is needed. If the oxygen is saturated with hemoglobin, or the arterial gases parameters are still below normal ranges, the requirement of tracheal intubation is evaluated as an urgent and transitory way to assure the U.A.
The first surgical strategies to deal with these patients were based exclusively on urgent maneuvers with aimed to save the patient’s life through therapeutically tracheotomy.
Tracheotomy procedures made in new born have a high morbidmortality rate. Some authors described 1% to 5% mortality rate [7]. Morbidity is related to tracheal stenosis and language development disorders, reaching a 50% rate of intellectual failure as a consequence [8]. It is important to highlight that a patient who suffers from Pierre Robin sequence must maintain the tracheotomy procedure until a safe airway is achieved, which, in expert’s considerations, is not until the patient is 2 to 4 years old [9].
In 1937 Callister [10] and later in 1949 Longmire [11] described a new technique of skeletal traction with orthopedic devices, with the main purpose of stimulating the mandibular growth and development without the need of osteotomies. Their reports showed good results in the management of the U.A. and weight gain after 2 and 3 months of treatment, however, no mandibular osteotomies were performed, instead, the forces applied were directly transmitted to the temporo mandibular joints, causing joint dislocations and sometimes even ankylosis.
In 1946 Douglas [12] describes a suture technique of the tongue and lip in order to manage the S.A. obstruction by the exclusive manipulation of the tongue’s position. Later, in 1982 Parsons [13] defines the two criteria which are the groundwork to justify the adhesion of the tongue to the lip in these types of patients; an inadequate weight/stature gain during 7 days and / or the maintenance of an endotracheal tube for longer than 3 days. He did not describe how long this junction had to be kept, or if any posterior alteration in feeding or fonoarticulation occurred. However, the main disadvantage of the technique is its lack in treating the etiologic factor of the sequence, keeping the patient with his micrognathia (Figure 1).
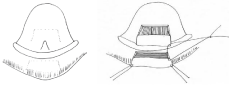
Figure 1: Lingual and lip flap.
In 1989 Delorme [14], proposed a new technique that consisted of the desinsertion of the suprahyoid muscles, with the aim of releasing the muscular traction of the mouth floor, which would be in a secondary way the responsibility the micrognathia [15]. In his work he shows 4 patients who received this treatment (Figure 2), one of which underwent an advanced mandible osteotomy, and another a gloso-lip suture procedure. Of the two cases which underwent muscular desinsertion only; one of them presented episodes of apnea.

Figure 2: Representation of Subperiostal release of the floor of the mouth
musculature. left, surgical approaches; Right, lingual reposition.
After analyzing the clinical outcomes obtained by this author and focusing on the primary problem, the micrognathia, muscular suprahyoid desinsertion did not appear to be a good therapeutic alternative for patients who suffer Pierre Robin sequence.
In a retrospective study, between 1964 and 1991, Caouette- Laberge [16] examined 125 cases with Pierre Robin sequence and who received various combinations of treatments with 13% of mortality and 23% of psychomotor delay in patients who survived.
In 1973 Snyder et al., in a preliminary study, achieved a canine mandibular enlargement through osteotomy and posterior bone traction, this being the first experimental study of osteogenic distraction in a facial skull [17].
During the 90´s, bone distraction begins to be practiced in the maxillofacial territory. McCarthy [18], Molina and Ortiz-Monasterio [19], and Guerrero [20] make the first efforts to achieve growth of bone and soft surrounding tissue. Through an osteotomy or corticotomy, a gradual distraction of bone structure is performed in order to achieve the expected bone enlargement. Technical concepts of osteogenic distraction described by Codivilla [21], and the biological announcements by Ilizarov [22] were key in the application of histyogenic distraction.
Patients and Methods
The group consists of 25 patients with Pierre Robin sequence and severe breathing problems (Table 1). Six of them required mechanical ventilation. All underwent mandibular distraction osteogenesis according surgical technique to be described later. The average age was 8.7 weeks (1.4 to 20). An average of 18.8mm of distraction was achieved, according to the need of each case in a range between 14 and 22 mm. All patients improved their respiratory parameters once completed the period of active distraction, keeping them in time with an average follow-up of 55.8 months (16-120 months).
Gender
age
Breathing disturbances
Mechanical ventilation
Amount of Distraction (mm)
Follow up (months)
Female
3 months
SAHOS + FP
Yes
20
120
Male
6 weeks
SAHOS
No
18
76
Male
2 months
SAHOS + FP
Yes
22
70
Male
10 days
SAHOS
Yes
20
70
Male
3 months
SAHOS
No
15
66
Female
4 weeks
SAHOS
No
18
66
Female
4 weeks
SAHOS + FP
No
20
64
Male
2 weeks
SAHOS
No
22
64
Female
3 months
SAHOS
No
16
62
Male
4 weeks
SAHOS + FP
No
20
60
Male
6 weeks
SAHOS + FP
Yes
22
60
Male
3 months
SAHOS
No
16
54
Male
2 months
SAHOS + FP
No
18
54
Male
3 months
SAHOS
No
15
54
Female
3 months
SAHOS + FP
No
14
52
Female
3 weeks
SAHOS +FP
No
16
50
Male
3 months
SAHOS
No
20
50
Male
3 months
SAHOS
No
22
48
Male
5 months
SAHOS
No
20
44
Male
3 months
SAHOS +FP
No
20
44
Female
6 weeks
SAHOS
No
18
42
Female
4 months
SAHOS
No
18
40
Male
2 months
SAHOS +FP
Yes
20
38
Male
2 months
SAHOS
Yes
20
30
Male
6 weeks
SAHOS
No
20
16
Male=17 Female= 8
Average: 8,736
Yes=6
Average: 18.8mm
Average:55,76 months
Table 1: OSAS + FP. (OSAS: obstructive sleep apneas syndrome; FP: feeding problems).
Case 1
An infant of 3 months old, with Pierre Robin’s sequence was admitted to the intermediate care unit, showing repeated episodes of obstructive apneas, which were handled initially with oxygen therapy. In a few days the respiratory obstruction worsened, causing the patient to be admitted into intensive care unit, where mechanical ventilation was required (Figure 3).
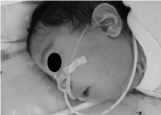
Figure 3: Infant of 3 months old with Pierre Robin Sequence.
A complete examination and study of the patient took place, which confirmed the diagnostic of Pierre Robin sequence including larynges- tracheal- bronchomalasia, permanent and severe bronchial obstructive syndrome, oxygen dependency, swallowing disruption, gastroesophagic reflux and chronic malnutrition.
An ostheogenic, bilateral mandibular distraction was proposed as a treatment with the aim of correcting the micrognathia and stimulating the suprahyoid muscles in order to gradually enlarge the U.A., which move correct the position of the tongue in the oral cavity. After the procedure was performed, the need of a gastrectomy and anti-reflux surgery would be evaluated.
Surgical technique
With the use of the patient’s profile radiography, mandible osteotomies were planned, vectors were traced and position of distractors was predicted.
Under general anesthesia, an intraoral approach on both mandibular body and ramus was made. Then, osteotomies on both mandibular ramus were performed, behind each angle, very carefully to avoid damage to the dental organs which were in intra-osseous evolution.
The osteotomized area was delimited using superficial corticotomy along all the external face of the mandible. Afterwards, osteotomy of the alveolar and basilar edges was performed, making a superficial vestibule cut, while being careful to preserve the inferior alveolar nerve (Figure 4).
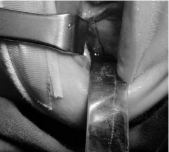
Figure 4: Mandible osteotomy behind of last bud.
A Kirschner wire was installed like Faria et al described [26], through both proximal segments, been carefully to do it completely horizontal (Figure 5). Then another Kirschner wire was installed and anchored from side to side of the front-inferior zone of the chin’s symphysis.
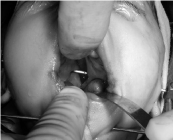
Figure 5: Kirschner wires.
Before the second Kirschner wire was installed, the skin between the wires was pinched in order to minimize a scar secuelae during distraction.
The external distractors Molina`s type (Wells Johnson Co) was installed at each mandibular side. Both sides were then activated until complete osteotomy was reached, returning the mandibular sides afterwards to their original position (Figure 6).
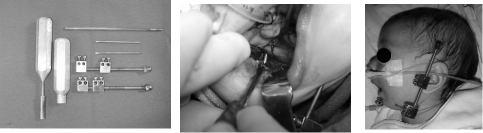
Figure 6: Left, distractor devices; Center, putting proximal screw; Right,
distractor device in position.
Three days later, activation was initiated with a 0.5mm magnitude (a complete turn of the distractor screw) every 12 hours during 20 days, obtaining 20mm mandibular distraction.
After the activation was complete, contention was performed for 4 weeks, keeping the distractor devices in a static position. Afterwards the distracter device was removed without the need of general anesthesia.
Results
25 patients were treated with this approach and modified anchor the external device. No releases of pin in any patients were seen. After the surgery, the patients were held in the intensive care unit. On the third day of osteogenic distraction, usually the extubation is possible, allowing spontaneous ventilation with the help of a nose mask with 50% oxygen. At the end of the first week the patients were moved to the intermediate care unit. During the period of distraction, suction was stimulated, and mandibular lateral movements were performed with the guidance of an operator (nurse), to stimulate growth and avoid ankylosis due to the pressure over the TMJ.
At the end of the activation period, the patients gained a normal respiratory physiology, with complete horizontal positioning of the tongue (Figure 7), and a larger U.A. area (Figure 8) with an average of 18.8mm of distraction. Gastroesophagic reflux was cured; witch was comprobated with a pH measurement. Adequate oral nutrition was achieved with the prior help of suction stimulation through a pacifier and feeding bottle. The maxillo-mandibular relation was corrected and proper chin projection, soft tissue coverage, and an adequate positioning of the alveolar bones was achieved in the immediate post distraction (Figure 9). These parameters were maintained throughout the follow-up time (average: 55.8 months).
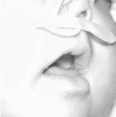
Figure 7: Horizontal position of the tongue.
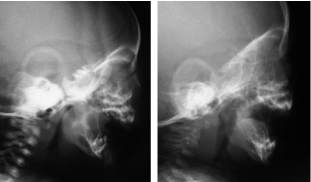
Figure 8: Left, preoperative radiography; Right, postoperative radiography.
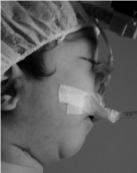
Figure 9: Preoperative profile.
The dental buds were preserved in all patients, and the alveolar nerve didn’t show any sensitive disturbs. In panoramic and teleradiographic images, the ramus and body of the mandible are anatomically normal, and upper air way was reestablished (Figure 10).
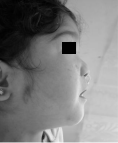
Figure 10: Postoperative profile.
Discussion
There are many ways to treat severe alterations of the U.A, tracheotomy being the most common treatment. The inconveniencies of this technique are high, increasing even more when performed in new born or very young children, causing high morbidity (tracheoesthenosis) and mortality [7,8,9].
Pierre Robin sequence has always been a hazard within the U.A. management due to the severe structural deficiency of the lower third of the face. When planning therapeutical strategies, we should always plan etiologic solutions that offer favorable clinical outcomes. Analyzing the situation from this point of view, when different therapeutical approaches are reviewed in the scientific literature, we can see that the consequences of the sequence are the ones confronted, rather than the etiological aspect of the disease. Pretending to recuperate the respiratory situation, without offering an adequate anatomically functional scenario, does not provide safe, stable or satisfactory results.
Skeletal traction techniques, without osteotomy, proposed by Callister [10], presented a severe aggression to the T.M.J., apart from being very uncomfortable for patients.
The technique which sutures the tongue and lip [12], improves ventilatory parameters in the short term, yet maintains the skeletal alterations, so there remains little space for tongue placement and interference with the mandibular growth. The musculature desinsertion of the mouth floor proposed by Delorme [14] does not improve the anatomical conditions of the micrognathia; no provide satisfactory clinical outcomes.
Distraction osteogenesis is a relatively new technique used to achieve mandibular enlargement. Based on bone physiology and biomechanical concepts, when an initial osteotomy and a posterior gradual traction are performed, we find a good solution for patients with alterations of the U.A. due to mandibular growth and less developed alterations.
McCarthy [18] and Molina [19], both propose an osteotomy in the angle zone of the mandibular ramus, installing pins carefully exactly to the line of fracture. We perform the osteotomy (the same as them) behind the last tooth bud in an oblique form, but we prefer to install a Kirshner wire, both, in proximal and in the mental zone [26]. We do this mainly for three reasons; the more anterior installation lowers the risk of damaging a tooth bud located in the mandibular body and the inferior dental nerve. Also, the more anterior the fixation of the device is, the distraction vector becomes more horizontal. The last reason is that it significantly diminishes the risk of displacement of the distractor device, because when inserted bi-cortically through the chin, achieving optimal stability. At the same time it does not interfere with the transversal growth of the chin, because being a smooth wire, it allows bone tissue to slide easily in a transversal manner.
Molina and Ortiz-Monasterio, propose the pin installation, first proximally and then distally. When using a Kirschner wire in the chin zone, we suggest installing it before the pin insertion, because in this order, the desired parallelism is easily achieved.
In our work, we started the distractor activation on the third day preceding surgery, with 1mm every 12 hours for the first three days, continuing with 0,5mm every 12 hours for the next 14 days. McCarthy [24], when presenting his 10 years of experience in distraction osteogenesis, mentioned that the latency period before initiating activation should be 5 days, keeping, and a rhythm every 12 hours during traction. He puts emphasis, in those patients too young, activation should be of 1,5mm daily, due to their great metabolic potential and the risk of premature consolidation. Molina [19] recommends 5 days as the latent period, after which he continues with distraction of 1mm every 24 hours. Denny [23], when presenting a series of 6 patients with obstructive airway disorders, to whom he had performed osteogenic distraction, started activation the next day of the intervention, with a rhythm of 2mm daily for the first 3 days. When analyzing the different reports, we decided to wait 3 days, in order to allow a clot to form in the osteotomized zone. Then activation was started with 1mm every 12 hours to allow a greater initial advance with the major aim of quickly dealing with the obstruction of the U.A. Distraction was every 12 hours rather than every 24 hrs, because this diminishes the separation magnitude of the two segments during each activation, lowering pain.
During the planning stage of the intervention it is fundamental to focus on the aim of the final treatment, keeping in mind that when dealing with patients in active growth, once the bone “wanted” enlargement is achieved, there exists a good probability that the future development of the skeletal piece diminishes. For this reason that many times it is preferable to achieve a major distraction, especially when patients present facial asymmetry and micrognathia [24].
Our priority in the treatment was to solve the respiratory problem, and secondly, solve the skeletal problem. That is the reason why the amount of total distraction was 20mm, which was determined in order to achieve proper ventilation, a harmonious look of the lower third of the face, and an adequate relationship between the alveolar processes (Figure 11,12).
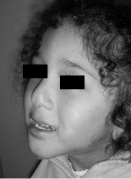
Figure 11: A four year follow up.
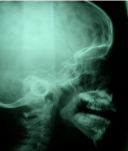
Figure 12: A teleradiography four years after.
Consolidation time is also a controversial subject in literature. Denny [23] proposes two days of contention for every day of distraction. Molina suggests putting an end to contention when you visualize the mature bone radiographically, approximately after 6 to 8 weeks [19]. Felemovicius [25] proposed a different time of consolidations depending on the age of the patient, based on osseous metabolism demonstrated by scintigraphy. In patients younger than one year, it only 4 weeks of contention phase is necessary [26].
References
- Robin P. Glossoptosis due to atresia and hypotrophy of the mandible. Am J Dis Child. 1934; 48: 541-547.
- Langman Jan. Embriología Médica Con Orientación Clínica, 9º edición. Editorial Médica Panamericana, Buenos Aires. 2005.
- Menasche V, Farrehi C, Miller M. Hipoventilation and cor pulmonale due to chronic upper airway obstruction. J. Pediatr. 1965; 67:198.
- Eliachar E, Duhamel B, Tassy R. [Apropos of the Pierre Robin syndrome]. Ann Pediatr (Paris). 1968; 15: 465-470.
- Bush PG, Williams AJ. Incidence of the Robin Anomalad (Pierre Robin syndrome). Br J Plast Surg. 1983; 36: 434-437.
- Dykes EH, Raine PA, Arthur DS, Drainer IK, Young DG. Pierre Robin syndrome and pulmonary hypertension. J Pediatr Surg. 1985; 20: 49-52.
- Zeitouni A, Manoukian J. Tracheotomy in the first year of life. J Otolaryngol. 1993; 22: 431-434.
- Singer LT, Kercsmar C, Legris G, Orlowski JP, Hill BP, Doershuk C. Developmental sequelae of long-term infant tracheostomy. Dev Med Child Neurol. 1989; 31: 224-230.
- Tomaski SM, Zalzal GH, Saal HM. Airway obstruction in the Pierre Robin sequence. Laryngoscope. 1995; 105: 111-114.
- Callister, A. Hypoplasia of the mandible (micrognathia) with cleft palate: Treatment in early infancy by skeletal traction. Am J Dis Child. 1937; 53:1057.
- LongMire WP Jr, SANFORD MC. Stimulation of mandibular growth in congenital micrognathia by traction. Am J Dis Child. 1949; 78: 750-754.
- Douglas B. The treatment of micrognathia associated with obstruction by a plastic procedure. Plast Reconstr Surg. 1946; 1: 300-308.
- Parsons RW, Smith DJ. Rule of thumb criteria for tongue-lip adhesion in Pierre Robin anomalad. Plast Reconstr Surg. 1982; 70: 210-212.
- Delorme R, Larocque Y, Caouette-Laberge L. Innovative surgical approach for the Pierre Robin anomalad: Subperiostal release of the floor of the mouth musculature. Plast. Recontr. Surg.1989; 83:960.
- Epois V. [Anatomy and development of the facial skeleton in labiomaxillopalatal clefts]. Chir Pediatr. 1983; 24: 240-246.
- Caouette-Laberge L, Bayet B, Larocque Y. The Pierre Robin sequence: review of 125 cases and evolution of treatment modalities. Plast Reconstr Surg. 1994; 93: 934-942.
- Snyder CC, Levine GA, Swanson HM, Browne EZ Jr. Mandibular lengthening by gradual distraction. Preliminary report. Plast Reconstr Surg. 1973; 51: 506-508.
- McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992; 89: 1-8.
- Molina F, Ortiz Monasterio F. Mandibular elongation and remodeling by distraction: a farewell to major osteotomies. Plast Reconstr Surg. 1995; 96: 825-840.
- Guerrero C. Intraoral distraction, en McCarthy, J Distraction of the craniofacial skeleton, Springer-Verlag New York Inc, 1999.
- Codivilla A. The classic: On the means of lengthening, in the lower limbs, the muscles and tissues which are shortened through deformity. 1905. Clin Orthop Relat Res. 2008; 466: 2903-2909.
- Ilizarov G, Lediov V, Shitin, V. The course of compact bone reparative regeneration in distraction osteosynthesis under different conditions of bone fragment fixation and experimental study (Russian). Exp Khir Anesteziol. 1969; 14: 3-12.
- Denny A, Kalantarian B. Mandibular distraction in neonates: a strategy to avoid tracheostomy. Plast Reconstr Surg. 2002; 109: 896-904.
- McCarthy JG, Katzen JT, Hopper R, Grayson BH. The first decade of mandibular distraction: lessons we have learned. Plast Reconstr Surg. 2002; 110: 1704-1713.
- Felemovicius J, Ortiz Monasterio F, Gomez Radillo LS, Serna A. Determining the optimal time for consolidation after distraction osteogenesis. J Craniofac Surg. 2000; 11: 430-436.
- Faria R, Castellón L, Nagelash E, Valladares S. A new way to anchor the external device in mandibular distraction: three case reports with a Pierre Robin sequence. Int J Oral Maxillofac Surg. 2011; 40: 471-474.