
Case Presentation
Austin J Otolaryngol. 2017; 4(1): 1089.
Radical Treatment of an Aggressive, Recurrent Benign Inflammatory Lesion of the Tongue: A Case Report & Review of the literature
Rachelle LeBlanc, Anil Sharma, Peter Spafford, Brent Wilde and Rick Jaggi*
Department of Otolaryngology-Head & Neck Surgery, College of Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
*Corresponding author: Jaggi R, Department of Otolaryngology-Head & Neck Surgery and Facial Plastic Surgery, College of Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
Received: April 05, 2017; Accepted: May 03, 2017; Published: May 10, 2017
Abstract
Background: There are limited publications on the management of large aggressive recurrent benign inflammatory lesions of the tongue. These lesions have a wide range of etiologies that must be explored. Many documented reports on eosinophilic ulcers/traumatic ulcers suggest surgical excision with recurrence rates relatively low.
Case Report: This article reports on an unusual case in a 48-year-old male patient with a large left anterolateral tongue ulcer with polypoid granulation tissue that has recurred after three attempts of partial glossectomy and injection of corticosteroids. A final left hemiglossectomy with a supraclavicular island flap reconstruction was performed and is currently being followed for ongoing management.
Conclusion: Aggressive, large benign inflammatory lesions of the tongue may benefit from radical treatment if all conservative management fails. Correct diagnosis, careful surgical planning, and patient preference of treatment should all be performed while maximizing success, quality of life, and improving tongue function.
Keywords: Case report; Traumatic ulcer; Eosinophilic ulcer; Pyogenic granuloma; Tongue; Hemiglossectomy
Abbreviations
CT: Computed Tomography; EU: Eosinophilic Ulcer; PG: Pyogenic Granuloma; TUG: Traumatic Ulcerative Granuloma
Introduction
Large tongue lesions in a non -smoker, non- drinker have a wide range of etiologies. The differential diagnoses include hyperplasias, papillomas, soft tissue tumor and malignancy, particularly squamous cell carcinoma. In the present case, histopathological investigations were essential to distinguish between a variety of benign inflammatory processes of the tongue. After initial investigations the differential diagnosis was narrowed to include Traumatic Ulcerative Granuloma (TUG), Eosinophilic Ulcer (EU) and Pyogenic Granuloma (PG).
TUGs/EUs are benign lesions that are usually self-limiting. They manifest as mucosal ulceration and inflammation that extends into underlying muscle, which shows a predominance of histiocyte-like mononuclear cells and eosinophils, in addition to nonspecific acute and chronic inflammatory cells [1]. The etiopathogenesis is unknown, however, trauma seems to play an important role in the development [2]. EUs are frequently located on the tongue but can also occur in other locations such as lips, buccal mucosa, palate, gingiva and floor of the mouth [3,4].
The other lesion that should be included in the differential diagnosis is PG, a hyperactive benign inflammatory lesion that most often occurs on the lips and mucosa of the oral cavity. PG lesions are exophytic, presenting with smooth or lobulated surfaces that usually bleed [5]. They typically range in size from a few millimeters to several centimeters, rarely exceeding 2.5cm [6,7]. The etiology of PGs is unknown, however, predisposing factors include chronic irritation, trauma, infections and hormonal factors [8]. PGs occur in a variety of ages, however, they are most common in the second decade of life. Women are affected more often, likely due to hormonal effects [9-11]. It is important to note that these lesions may recur if not removed completely [5].
The following case report describes an aggressive recurrent large benign inflammatory lesion treated with three attempts of partial glossectomy, intralesional corticosteroid injection and a hemiglossectomy with a supraclavicular island flap reconstruction.
Case Presentation
A 48-year old male presented to the Otolaryngology- Head and Neck Surgery department with a 6-week history of a left tongue lesion described as quite painful. He had a history of recurrent abscess ulcerations, which resolved after 2 weeks. He is a non-smoker, nondrinker with no significant risk factors for any cancer. Initial physical exam showed a 1 x 2cm ulcerative lesion along the left lateral tongue, which was firm on palpation. No other lesions noted on the entire sub sites of the oral cavity. Endoscopic laryngoscopy was performed and was completely normal with no base of tongue involvement.
Computed Tomography (CT) of the neck with contrast was performed. Findings suggested an asymmetric, ill-defined round region of hypodensity measuring 22x19mm at the left base of tongue. There was no evidence of local extension, with no crossing over the midline. A left partial glossectomy was performed and sent to surgical pathology. The specimen consisted of squamous lined mucosa with a large central ulceration. The squamous epithelium, at the margins of the ulceration, exhibited hyperkeratosis and parakeratosis. Staining with pankeratin showed no evidence of invasive carcinoma. Immunohistochemistry and special stains did not highlight infectious organisms.
Three months later the patient returned to the department with a recurrent mass on the left lateral margin of the tongue, which was painful with intermittent bleeding. Further physical examination revealed an exophytic mass along the left lateral margin of the tongue where the previous resection had taken place. There was no active bleeding and was soft on palpation. A second resection was performed and the specimen was sent to surgical pathology. The specimen consisted of several sections of polypoid mass. The mass was lined with squamous epithelium, which in some places, exhibited marked pseudo-epitheliomatous hyperplasia. In focal areas, the squamous mucosa was ulcerated with a fibropurulent cap and underlying granulation tissue. The inflammatory infiltrate consists primarily of neutrophils with a secondary population of lymphocytes, macrophages and plasma cells. Eosinophils were also noted within the areas of ulceration and granulation tissue. At this time the findings suggested a diagnosis of an ulcer with polypoid granulation tissue. It did not represent a pyogenic granuloma, as it did not have characteristic lobular architecture of the lesion.
Two months later the patient was referred for a second opinion with another recurrence of the left lateral granulomatous tongue ulcer. The lesion returned with more exuberance and pain, and was bleeding. Attempt with steroid injections into the base of the granulation tissue was made and had little effect. Further examination revealed tongue deviation significantly to the left upon protrusion, implying a left hypoglossal nerve paralysis. The patient was taken back to the operating room for an excision of the left posterior floor of mouth lesion and a partial glossectomy was performed. The surgical wound was left open to heal by secondary intention (Figure 1-3). Surgical pathology revealed left tongue ulcer with extensive inflammation.

Figure 1: Day before third attempt of partial glossectomy.

Figure 2: Post-op third attempt of partial glossectomy.

Figure 3: One month post-op third attempt of partial glossectomy.
The patient returned again and was referred for a third opinion with the supervising author (RJ) two weeks after the third attempt of partial glossectomy with further recurrence. The lesion completely regrew larger than previous, involving the anterior two-thirds of the left lateral tongue (Figure 4). Endoscopic laryngoscopy showed no involvement of the base of tongue. The patient experienced significant discomfort with pain radiating to the ear, dysphagia, difficulty with speech and intermittent bleeding. The decision to perform a left hemiglossectomy with supraclavicular island flap reconstruction was made (Figure 5) and the specimen was sent to surgical pathology. Final pathology revealed ulcerated squamous mucosa with granulation tissue. The squamous mucosa showed ulceration, leaving a large bed of granulation tissue. Much of the deeper inflammation was chronic in nature, rich in plasma cells and lymphocytes. There was mild atrophy and chronic inflammation involving the underlying minor salivary gland and patchy degeneration of the intrinsic skeletal muscle, which could be attributed to secondary alterations due to the proximity to the bed of granulation tissue. There was no evidence of dysplasia or malignancy. Neither vasculitis nor granulomatous inflammation was seen.
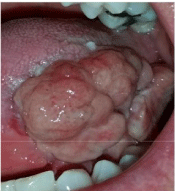
Figure 4: Two months post-op third attempt of partial glossectomy.

Figure 5: Post-op hemiglossectomy with supraclavicular island flap
reconstruction.

Figure 6: H&E Staining: A) Low power field of exuberant exophytic and
endophytic growth. B) Higher power showing neoangiogenesis, inflammation
including eosinophils, and prominent fibroblastic proliferation.
Discussion
There are many underlying causes of ulcerative tongue lesions (Table 1). In the present case, traumatic ulcer, eosinophilic ulcer and pyogenic granuloma are the most likely etiologies. Most benign inflammatory lesions are self-limiting if the causative agent is removed. However, in the present case, it was difficult to identify the causative agent as the patient denied a history of tongue trauma, infection, smoking and alcohol use. Although the patient denied of any trauma history, the histopathology reviled abundant inflammatory cells most in keeping with a traumatic etiology. More specifically, results pointed to an eosinophilic ulcer and/or traumatic ulcer.
Eosinophilic
Ulcer
Etiology
Description/
Clinical Features
Histopathology
Treatment
Trauma
Manifests as a rapidly developing solitary ulcer, with elevated and indurated borders arising in the oral cavity.
Polymorphic inflammatory infiltrate
extending deep into the submucosa, underlying muscle and salivary glands.
Self-limiting Excision Intralesional
corticosteroids
Reactive/Inflammatory
May show peripheral erythema, white or yellowish base and fibrinous membrane on the surface.
Numerous eosinophils and large histiocytic cells with pale nuclei and
frequent mitoses, in some instances.
Topical Steroids
Biting injuries
Lesions may be asymptomatic or
extremely painful and can mimic oral
cancer clinically.
showing a pseudolymphomatous
aspect, are characteristic.
Topical
antibiotics
Traumatic Ulcer
Denture irritation, biting
injuries, burns and friction
irritation from sharp or
fractured teeth.
Localized area on the skin or mucosa in
which the surface epithelium has been
destroyed.
Surface ulceration covered by a
fibrinopurulent membrane consisting of acute inflammatory cells intermixed with fibrin.
Removal of
causative agent.
Shape and size are variable.
The stratified squamous epithelium
from the adjacent surface may be
hyperplastic and exhibit areas of
reactive squamous atypia.
Relief of pain with topical
agents.
Usually painful and of short duration.
The ulcer bed is composed of a
proliferation of granulation tissue with
areas of edema and an infiltrate of
acute and chronic inflammatory cells.
Pyogenic
Granuloma
Unknown.
Red, nodular overgrowth of granulation
tissue that arises from mucosal or skin surfaces.
Pedunculated lesion composed of
granulation type tissue separated by bands of connective tissue.
Conservative
excision. They
may recur.
Mild trauma, hormonal factors and infection are prominently mentioned.
? of oral lesions are found on gingival followed by lips, tongue, buccal mucosa, palate, vestibule and edentulous areas.
Bleed easily and mild pain.
Covering epithelium almost meets at base of lesion; has lobular arrangement of capillaries at base.
Lobules consist of discrete clusters of endothelial cells with indistinct to
prominent lumina.
Irritation
Fibroma
Trauma to affected mucosa. Accidental biting accounts for most of these lesions.
Dome-shaped soft tissue mass usually found on buccal mucosa along the line of occlusion. Less frequently found on the lips and tongue.
Fibrous hyperplasia that is
collagenous and acellular.
Excision
Squamous Cell
Carcinoma
Unknown.
Early carcinoma may clinically appear as leukoplakia or erythroplasia. Another
common clinical appearance is an area of chronic ulceration.
Loss of basement membrane.
Surgical excision
and possible
irradiation.
Smoking and alcohol are risk factors, and human papilloma virus is suspected.
Certain areas are more vulnerable; soft palate, lateral and ventral tongue mucosa, and floor of mouth.
Variable degree of keratinization, pleomorphism and mitotic activity
Chemotherapy is adjunctive at this time.
Table 1: Differential diagnosis of tongue ulcerations and lesions.
Eosinophilic ulcer of the oral mucosa is a benign inflammatory lesion with uncertain etiology, pathogenesis, and treatment. There have been other terms to describe it reported in the literature such as, traumatic ulcerative granuloma with stromal eosinophilia, eosinophilic granuloma of tissue and atypical histiocytic granuloma [12-14]. Popoff first described this benign inflammatory ulcer in 1956 and in 1970, the lesion was proposed as a distinct entity by Shapiro and Juhlin [15-17].
Although it is not entirely proven, Segura and Pujo suggest that EUs are traumatic in origin [12]. Though the patient in the present case denied trauma, it could be speculated that accidental bites or trauma through mastication occurred unconsciously. Vélez, et al. have suggested that the only contributing factor to the development of EUs is trauma and could potentially lead to viral or toxic agents entering the underlying tissue causing an inflammatory response [16].
Histopathology for EUs reveals poorly formed granulation tissue showing an increased number of capillaries with predominantly endothelial cells [2]. Inflammatory infiltrate of the specimen is composed of small round lymphocytes, abundant polymorphonuclear eosinophils and other inflammatory cells such as neutrophils, plasma cells and histiocytes [2]. In our case, this composition of cells was shown on histology making the diagnosis of EU/TUG most likely.
There are many different treatment modalities used for EU/TUG. Options include observation, antibiotics, systemic corticosteroids, curettage, cryosurgery and surgical excision [2]. It has been frequently reported that with surgical incision/excision there has been no further local recurrence [2]. The present case was atypical in that the lesion recurred three times before a radical approach was taken.
The present benign inflammatory lesion in this case exceeded 2.5cm and was rapidly growing to an uncomfortable size. After review of literature the decision was made to take an aggressive surgical approach. Radical wide excision is usually reserved for malignant disease. In the present case, the lesion relapsed three times after conservative surgical excision and had little response to intralesional corticosteroid injection. Due to the aggressive nature of the lesion’s recurrence after previous conservative surgical excisions, along with the patient’s pain, nerve palsy and bleeding, a left hemiglossectomy with a supraclavicular island flap reconstruction was performed.
Conclusion
An aggressive recurrent benign inflammatory lesion was treated by three attempts of partial glossectomy and finally eradicated by a hemiglossectomy with a supraclavicular island flap reconstruction. Since the present case was aggressive in nature after failed attempts of conservative treatments, a radical approach may be a beneficial treatment option. If the lesion is large and rapidly growing, exceeding more than 2.5cm, radical treatment may be considered. Correct diagnosis, careful surgical planning, and patient preference of treatment should all be taken into consideration while maximizing success, quality of life, and improving tongue function.
Authors’ Contributions
RL performed a chart review, data collection and drafted the manuscript. AS and PS collected the photographs and submitted data for the report. BW provided the pathology and helped draft the pathology of the manuscript. RJ conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approve the final manuscript.
References
- Almazrooa SA, John O, Sook-Bin W. Unusual large tongue ulcer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 116: 4-8.
- Chandra S, Raju S, Sah K, Anand P. Traumatic ulcerative granuloma with stromal eosinophilia. Arch Iran Med. 2014; 17: 91-94.
- Eleni G, Panagiotis S, Andreas K, Georgia A. Traumatic ulcerative granuloma with stromal eosinophilia: a lesion with alarming histo-pathologic presentation and benign clinical course. Am J Dermato-pathol. 2011; 33: 192-194.
- Segura S, Pujol RM. Eosinophilic ulcer of the oral mucosa: a Oral Dis. 2008; 14: 287-295.
- Ghalayani P, Hajisadeghi S, Babadi F. Extragingival pyogenic granuloma associated with medication: Report of an unusual case. Dental Research Journal. 2014; 11: 400-404.
- Bouquot JE, Nikai H. Lesions of the oral cavity. In: Gnepp DR, editor. Diagnostic surgical pathology of the head and neck. 3rd ed. Philadelphia: WB Saunders. 2002; 141-233.
- Verma PK, Srivastava R, Baranwal HC, Chaturvedi TP, Gautam A, Singh A. Pyogenic Granuloma - Hyperplastic Lesion of the Gingiva: Case Reports. The Open Dentistry Journal. 2012; 6: 153-156.
- Pitarch G, Perez-Ferriols A, Millan F. Recurrent pyogenic granuloma. Actas dermosifiliogr. 2012; 103: 536-539.
- Neville BW, Damm DD, Allen CM, Bouquot JE. 2nd ed. Philadelphia: WB Saunders; 2002. Oral and maxillofacial pathology. 437-495.
- Regezi JA, Sciubba JJ, Jordan RCK. 4th ed. Philadelphia: Oral pathology: Clinical pathologic considerations. 2003; 115-116.
- Eversole LR. 3rd ed. Clinical outline of oral pathology: Diagnosis and treatment. 2002; 113.
- Segura S, Pujol RM. Eosinophilic ulcer of the oral mucosa: a Oral Dis. 2008; 14: 287-295.
- Boffano P, Gallesio C, Campisi P, Roccia F. Traumatic ulcerative granuloma with stromal eosinophilia of the retromolar region. J Craniofac Surg. 2009; 20: 2150-2152.
- Ficarra G, Prignano F, Romagnoli P. Traumatic eosinophilic granu- loma of the oral mucosa: a CD30+(Ki-1) lymphoproliferative dis- order ? Oral Oncol. 1997; 33: 375-379.
- Elovic AE, Gallagher GT, Kabani S, Galli SJ, Weller PF. Wong chronic oral ulcers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996; 81: 672- 681.
- Vélez A, Alamillos FJ, Dean A, Rodas J, Acosta A. Eosinophilic ulcer of the oral mucosa: report of a recurrent case on the tongue. Clin Exp Dermatol. 1997; 22: 154-156.
- Shapiro L, Juhlin EA. Eosinophilic ulcer of the tongue report of two cases and review of the literature. Dermatologica. 1970; 140: 242-250.