
Review Article
Austin Pancreat Disord. 2017; 1(1): 1002.
Marine Sea Cucumber Saponins and Diabetes
El Barky AR*, Ali EMM and Mohamed TM
Biochemistry Division, Chemistry Department, Faculty of Science, Tanta University, Tanta, Egypt
*Corresponding author: Amira Ragab EL Barky, Department of Biochemistry, Faculty of Science, Tanta University, Egypt
Received: February 02, 2017; Accepted: February 20, 2017; Published: February 22, 2017
Abstract
Diabetes mellitus (DM) is a metabolic disorder that characterized by hyperglycemia. DM resulted from defects in insulin secretion, action, or both of them. The chronic hyperglycemia can lead to diabetic complication which is considered as a major cause of morbidity and mortality. Marine invertebrates, sea cucumber have an impressive profile of valuable bioactive compounds, for instance, holothurians that exhibit a wide range of biological activities and have many therapeutic effects. This review highlights the valuable bioactive saponin on the complication of diabetes.
Keywords: Diabetes; Saponin, Hyperglycaemia; Sea cucumber
Abbreviations
DM: Diabetes Mellitus; IL-6: Interleukin-6; STZ: Streptozotocin; TNF-α: Tumor Necrosis Factor Alpha
Introduction
Diabetes mellitus (DM) is a chronic metabolic disorder that characterized by hyperglycemia. It is the most common disease among patients with pancreatic cancer and chronic pancreatitis. Exocrine pancreatic insufficiency is extremely associated with diabetes, with high prevalence in both types I and II. The incidence of diabetes caused by exocrine pancreatic disease appears to be underestimated and may comprise 8% or more of the general diabetic patient population [1]. DM resulted from defects in the pancreas where insulin secretion is not produced enough or cells do not respond to the insulin that is produced or both of them, resulting in high blood sugar action. The chronic hyperglycemia resulted from diabetes can lead to irreversible damage, dysfunction and failure of various organs [2]. Diabetes is classified into four categories (Figure 1)[2]. The major two categories which are common to all of the people is Type I is also known as Insulin-dependent diabetes mellitus, which is identified by absolute insulin reduction. The main causes of type I diabetes are immune or idiopathic causes [3], whereas Type II diabetes the second category is known as noninsulin-dependent diabetes mellitus, it is a challenging metabolic disorder. Its etiology is related to several causes, for instance, a significant loss of insulin producing, beta cell mass via advanced programmed cell death and disrupted cellular autophagy. There is also strong index that β cells are dynamically active cells, which, under specific conditions such as obesity, can increase in size and thus increase insulin secretion in type 2 DM [4].
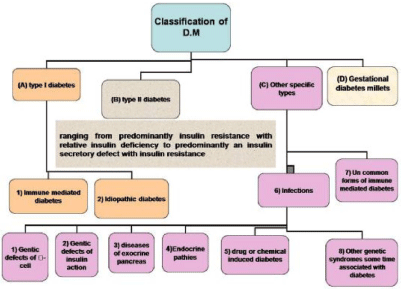
Figure 1: Classification of diabetes mellitus [2].
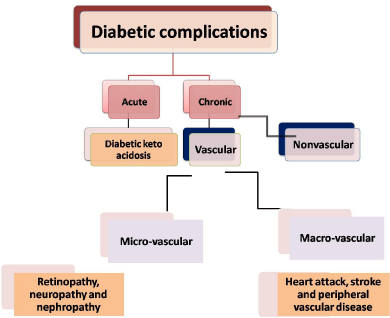
Figure 2: Diabetic complication.
Free Radicals and Diabetic Complications
The complications of diabetes were classified as acute complications like diabetic ketoacidosis and chronic complications. Chronic complications are subdivided to vascular and nonvascular complications. The vascular complications are also, divided into microvascular and macrovascular complications [5] figure (2). Oxidative stress is the main cause of the etiology of diabetic complications as it resultant in micro-vascular (retinopathy, neuropathy and nephropathy) and macrovascular (heart attack, stroke and peripheral vascular disease) complications which is consider the major cause of morbidity and mortality [6,7]. Oxidative stress is increased in DM, due to the increased level production of oxygen free radicals and decreased the level of the antioxidant defense mechanisms. The conclusion of the increased level of oxygen free radicals lead to lipidperoxidation of the cellular structures, the lipidperoxidation is thought to play an important role in atherosclerosis and the microvascular complications [8]. Free radicals are formed in diabetes as a result of glucose autoxidation, polyol pathway and non-enzymatic glycation of proteins [9]. The increased level of free radicals and decreased level of antioxidant defense lead to high level of lipidperoxidation, spoilage of the cellular organelles, hinder the enzymes activities and lead to diabetic complications [10].
Regardless of the presence of anti-diabetic medicines, screening for new anti-diabetic sources from natural products is still attractive as they contain substances that have a safe effect on diabetes mellitus. Natural compounds supposedly to be suitable alternatives for diabetes therapy. They may minimize the risk of the disease. Large amounts can be consumed in daily, which is a positive aspect [11].
Sea Cucumber, Echinoderms belong to a phylum of marine invertebrates that have about 6000 living species classified into five classes: Crinoidea, Holothuroidea, Echinoidea, Asteroidea and Ophiuroidea. The extract of these marine invertebrates have been shown to numerous biological activities such as antibacterial, antifungal, antiviral, antitumor and anti-coagulant, this bioactive compound has made them an attractive source [12]. Sea cucumbers have long been recognized in the folk medicine in addition to their high nutritious value, they could nourish the body, tonifying kidney, moistening dryness of the intestine, treatment of stomach ulcers, asthma, hypertension, rheumatism, pain, gout, asthtma, eczema, hyperglycemia, hypertension and wound healing [13,14]. They have the ability to reduce the growth of cancer cells [15]. One of the contents of sea cucumber that have numerous biological activity is saponin. Sea cucumber saponin is distributed in the body wall, internal organs, and glands of the marine invertebrate sea cucumber [16].
Holothuroid Saponins
Saponins identified as holothurians are the main bioactive compounds of sea cucumber that exhibit a wide range of biological activities and have many therapeutic effects [17]. The name is derived from the latin word “sapo” which means soap, Because when saponins are shaken with water, they tend to form soap-like foams. Sea cucumber saponins are primarily triterpene glycosides of lanosterol-type aglycone with a saccharide moiety attached at the C-3 position [18]. Saponins vary in the amount of sapogenin and in the lengths, linkages, and substituents of their sugars [19]. The aglycone part, designated as genin or sapogenin that is an either triterpenoid (C-30) or neutral or alkaloid steroids (C-27) [20]. The aglycone part covalently linked to one or more monosaccharide sugar moieties [21]. The monosaccharide sugar may by glucose, galactose, glucuronic acid, xylose, rhamnose the oligosaccharide is attached at the C3 position but some types of saponins have more than one sugar which is attached to the C26 or the C28 positions [22]. So, the aim of this article review is to show the mechanism of action of sea cucumber saponin on amelioration of some biochemical parameters in diabetic rats. It is hoped that the information will provide the reader with information regarding the anti-diabetic potential of saponins which has been extracted from sea cucumber and stimulate further research into these marine compound.
Serum Adiponectin Level
Adiponectin, a 30-kDa protein mainly secreted by adipocytes [23]. Adiponectin regulates glucose metabolism through stimulation of adenine monophosphate-activated protein kinase (AMPK) (Yamauchi et al., 2002) and increases muscle fat oxidation and glucose transport mediated through inhibition of acetyl-CoA carboxylase [24]. Also, adiponectin has been found to decrease the expression of phosphoenolpyruvate carboxylase and glucose-6- phosphatase, leading to inhibition of hepatic gluconeogenesis [25]. Further, the activation of peroxisome proliferator- activated receptor (PPAR)-α leading to decreased level of triglyceride in skeletal muscles and liver [26]. Furthermore, adiponectin is also an essential mediator for many therapeutic benefits of the PPARγ, including insulin sensitization and vascular protection [27]. Schalkwijk [28] reported that serum adiponectin levels were increased in people with type I diabetic as well as in patients with genetically defective insulin receptors who have microvascular complications. Moreover, EL Barky [29] showed that rats that intraperitoneal injected with STZinduced diabetes significantly increase serum adiponectin level in their blood as compared to the control normal group. The increase in the concentration of total adiponectin was mostly caused by a major excess of the dodecameric or high molecular mass (HMW) sub-form. This association was not related to gender or diabetic nephropathy status [30]. HMW oligomer may be the main biological active form that concerning glucose homeostasis, whereas the central actions are related to the low molecular weight oligomers [31]. The increment in the adiponectin levels in patients with type 1 diabetes appears to be highly related to the long of diabetes duration, irrespective of the metabolic control. Among other factors, a putative role for residual beta-cell function in the arranging of circulating adiponectin levels can be considered [32]. In a study by EL Barky [29] they evaluated the effect of sea cucumber saponin on STZ-diabetic rats, they reported that sea cucumber saponin resulted in a significant decrease in serum adiponectin concentration in the serum of diabetic rats that received saponin daily as compared to the diabetic non-treated group. The main mechanism by which adiponectin enhances insulin sensitivity appears to be due to improved lipid and glucose metabolism [33] figure (3).
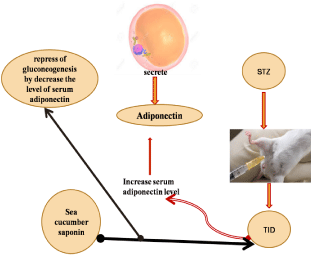
Figure 3: The mechanism action of sea cucumber saponin on STZ-induced diabetes rats.
Serum Proinflammatory Marker Level
Inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) has been involved in the pathogenesis of diabetes mellitus [34], it catalyzes multiple signaling cascades which resultant in β-cell apoptosis [35] in T1D. In addition, IL-6 is a pleiotropic cytokine has an influence on the pathogeneses of obesity, insulin resistance, β-cell destruction and both of type I and type II diabetes [36] Both of proinflammatory cytokines TNF-a and IL-6 are produced by infiltrating macrophages, lymphocytes and monocytes, destroy the pancreatic β-cells and created type-1 DM via enhancing the formation of oxygen free radicals, lipid peroxides and aldehydes [37]. Meanwhile, oral sea cucumber saponin extract administration to STZ-diabetic rats resulted in a significant reduction in the values of serum IL-6 and TNF-a concentrations. Saponins can prevent the lipopolysaccharide-induced production of TNF-a by blocking transcription factor NF-KB (nuclear factor kappa-light-chainenhancer of activated B cells) which regulates the transcription of many genes associated with inflammation [38].
Serum Alpha-amylase Activity
The increasing activities of serum pancreatic enzyme suggest an inflammation of the exocrine pancreas, be known as pancreatitis [39]. α-amylase is one of the main enzymes produced in the exocrine pancreatic cells, perhaps known as an adequate indicator of organ’s activity in both physiological and pathological states [40]. The increment in the α -amylase activity in diabetes may point to the release of the enzyme from cellular compartments which caused by increment of damaging processes [41]. Exocrine pancreatic insufficiency has been seen in diabetic patients [42]. Insulin contributes to the regulation of acinar cell function, supported by the presence of insulin receptors on acinar cells [43]. Serum alpha-amylase activity increases according to the grade of hyperglycemia [44]. Alpha-Amylases hydrolyze complex polysaccharides to yield oligosaccharides and disaccharides which are then hydrolyzed by α-glycosidase to monosaccharide which is absorbed through the small intestines into the hepatic portal vein and increase postprandial glucose levels [45]. Natural α-amylase inhibitors have been demonstrated to be beneficial in reducing postprandial hyperglycemia by slowing down the digestion of carbohydrates and, consequently the absorption of glucose. Saponins have also been known to be α-amylase inhibitor [46]. For instance, Holothuria thomasi, sea cucumber saponin has significantly lowerd the activity of serum alpha-amylase in STZ-induced diabetes in rats [29]. Triterpenoids saponins are known to cause insulin like effects and hinder the formation of glucose in the blood stream, which may be helpful in the treatment of diabetes [47]. The mechanism of inhibition of the glycolytic activity of α-amylase may occur through the direct blockage of the active center at several sub sites of the enzyme as also suggested for other inhibitors [48]. So, saponin extract act as α-amylase inhibitor.
The pancreas is the primary source of serum lipase. Lipase (triacylglycerol acylhydrolase) can catalyze the ester bonds hydrolysis on the glycerol backbone of the lipid substrate. Increased the activities of serum lipase have also been linked with pancreatitis, pancreatic duct obstruction, pancreatic cancer, and other pancreatic diseases [49,50]. Pancreatitis is classified as Type 3c diabetes, according to the American diabetes association [51]. The high activated of serum lipase can be compounded in another disease rather than pancreatitis, for instance, hyperglycemia and ketoacidosis [49,50,52].
Lipase, protease and amylase enzymes are important in managing diabetes as they will help digest all three groups of nutrients: proteins, fats and sugars [53]. Saponins which have been extracted from sea cucumber species inhibited pancreatic lipase and slow the absorption of TGs and cholesterol [54]. The latter effect was confirmed by an increase in the fecal excretion of neutral lipids and cholesterol in the rats whose diet had been supplemented with saponin [55].
Serum and Liver Total Cholesterol, Triacylglycerols and VLDL-C Levels
Hyperlipidemia, are implicated in the development of microvascular complication of diabetes, which are the major causes of morbidity and death [56]. Diabetes mellitus is extremely associated with impaired lipid metabolism such as high level of total cholesterol, triglycerides and abnormalities in serum lipoproteins [57] hypercholesterolemia in the STZ-induced diabetic rat’s created from the increased intestinal absorption and synthesis of total cholesterol [58]. Natarajan and Nadler [59] stated that monocyte adhesion to endothelial cells as well as that excessive proliferation and migration of vascular smooth muscle cells (VSMC) are key in the development of atherosclerosis in diabetes. Synthesis of VLDL is enhanced by an increase in the flow of free fatty acids in liver and finally, the particles are converted to low-density lipoprotein (LDL). Some studies revealed that increased levels of VLDL as a consequence of decreased clearance and also over-production in type 1 DM subjects. The increased circulatory VLDL-C and the associated triglycerides due to defective clearance of these particles from circulation [60], these changes were attributed to the altered activity of lipoprotein lipase. Elevated plasma triglyceride concentration is seen in type1 DM and type 2 DM either due to triglyceride over-production and /or underutilization. Lipoprotein lipase activity is markedly impaired. Insulin enhances lipid synthesis and suppresses lipid degradation by stimulation of transcription factors such as steroid regulatory element-binding protein (SREBP)-1c in the liver and in adipose tissue [61].
On the other hand, untreated diabetic rats will be prone to decrease the lipid contents in their blood and liver [29]. The decrease of lipid profile may be due to rats need more energy rather than glucose so, it broke down lipids to obtain energy which in agreement with EL Barky [29]. Sea cucumber saponin has been reported to decrease serum and liver TC, TAG and VLDL. Moreover, Hu [62] reported that Pearsonothuria graeffei, sea cucumber could markedly reduce hepatic lipids accumulation as well as serum TAG and TC concentration. The rats exhibited slight decrease tendency in both serum TG and TC, even if fed at 0.01% sea cucumber saponin (SSC); when added 0.03% and 0.05% SSC, the rats had much lower lipids level in serum. It is suggested that dietary SSC could reduce serum lipids in a dose response-manner. They concluded that the lipidslowering effect of dietary SSC may be partly associated with the enhancement of β-oxidation via PPAR α activation. Saponins are known antinutritional factors, which decrease the level of cholesterol by binding with cholesterol in the intestinal lumen, preventing its absorption and /or by binding with bile acids, causing a reduction in the enterohepatic circulation of bile acids and increase its fecal excretion [63] Saponins improve glucose and lipid homeostasis by restoring the deregulated glycolytic and gluconeogenic enzymes in the diabetic state through the activation of AMP-Activated protein kinase (AMPK) which plays a fundamental role for adjusting carbohydrate and fat metabolism [64]. So, saponin is known to lower triglyceride by inhibiting the pancreatic lipase activity.
Alpha-glucosidase
Alpha-glucosidase located in the brush border of the small intestine that acts upon α (1?4) bonds. α-Glucosidase can release glucose by hydrolyzing linear and branched isomaltose oligosaccharides, resulting in postprandial hyperglycemia [65]. Sea cucumber saponin (SCS) inhibited yeast as well as that rat intestinal α-glucosidase activity in a dose-dependent manner and showed a better inhibition of yeast α-glucosidases as compared to the positive control. SCS treatment amended the oral glucose tolerance in highfat- diet-fed mice [62]. The inhibitory effect of SCS on α-glucosidase is been likely to contribute to this effect [18].
Serum Glucose and Insulin Level
Streptozotocin (STZ) is a chemical substance that produces pancreatic islet β-cell destruction and is mostly used to produce a model of type 1 DM in experimental studies [66]. Streptozotocin was preferred to induce diabetes in rats rather than alloxan. STZ is a toxin with the ability to induce selective destruction of pancreatic beta cells that resulting in decrease serum insulin level and elevated blood glucose [67]. STZ causes a notable reduction in insulin release by the destruction of pancreatic β–cells [68]. Streptozotocin is analogous the glucose and N-acetyl glucosamine. STZ is taken up by the pancreatic β-cells through the GLUT 2 transporter wherever it causes destruction and finally death of β-cell islets by DNA fragmentation due to the nitrosourea moiety.
There are three major pathways associated with cell death are: (I) methylation of DNA by the formation of carbonium ion (CH3+) resulting in the activation of the nuclear enzyme poly-ADP-ribose synthetase as part of the cell repair mechanism and therefore cause NAD+ depletion; (ii) free radical generation as hydrogen peroxide and (iii) nitric oxide production [69].
Sea cucumber saponin significantly reduced serum glucose level and increase serum insulin level in streptozotocin diabetic [29]. Sea cucumber saponins have been reported to decrease blood glucose levels in different mechanisms such as regeneration of insulin action via increased plasma insulin levels and release insulin from the pancreas. It modulates insulin signaling by decreases serum TNF-α and IL-6 level. Suppress the activity of disaccharide which was confirmed by decreasing the activity of serum-alpha amylase activity. It activate glycogen synthesis by increasing liver content of glycogen and repress of gluconeogenesis by decrease the level of serum adiponectin [2, 29] figure (4).
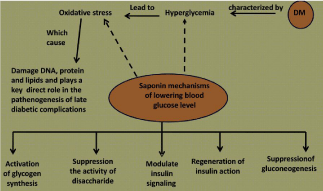
Figure 4: Mechanism action of saponin in diabetes [2].
Conclusion
This article review has abbreviated the fundamental role of sea cucumber saponin as an antidiabetic agent. Saponins from marine animals have been reported to have a hypoglycemic activity. Saponins have been reported to decrease blood glucose levels in different mechanisms such as regeneration of insulin action via increased plasma insulin levels and release insulin from the pancreas. Suppress the activity of disaccharide which was confirmed by decreasing the activity of serum alpha amylase activity. It activates glycogen synthesis by increasing the liver content of glycogen and repress of gluconeogenesis by decrease the level of serum adiponectin. Thus, exploring the therapeutic potential of saponin which has been extracted from different species of sea cucumber on the diabetic patient will benefit millions of people who suffer diabetes and complication that result from hyperglycemia.
References
- Rahman MH, Ali MY. Pancreatic Disorders and Diabetes Mellitus. Faridpur Med. Coll. J. 2015; 10: 36-39.
- El Barky AR, Hussein SA , Alm-Eldeen AA, Hafez YA, Mohamed TM. Saponins and their Potential Role in Diabetes Mellitus. Diabetes Management. 2017. Under press.
- Martha S. Pancreatic Hormones anti diabetic Drugs, in Basic & Clinical Pharmacology. 2007.
- Marrif HI, Al-Sunousi S. Pancreatic β Cell Mass Death. Frontiers in Pharmacology. 2016; 7: 83.
- Tripathi BK, Srivastava AK. Diabetes mellitus: complications and therapeutics. Med Sci Monit. 2006; 12: 130-147.
- Vanderjagt DJ, Harrison JM, Ratliff DM, Hunsaker LA, Vanderjagt DL. Oxidative stress indices in IDDM subjects with and without long-term diabetic complications. Clin Biochem. 2001; 34: 265-270.
- Patel DK, Kumar R, Prasad SK, Sairam K, Hemalatha S. Antidiabetic and in vitro antioxidant potential of Hybanthus enneaspermus (Linn) F. Muell in streptozotocin-induced diabetic rats. Asian Pac J TropBiomed. 2011; 1: 316-322.
- Soliman GZA. Blood lipid per oxidation (superoxide dismutase, malondialdehyde, glutathione) levels in Egyptian type 2 diabetic patients. Singapore Med J. 2008; 49: 129-136.
- Obrosova IG, Vanteysen C, Fathallah L, Cao X, Greene DA, Stevens MJ. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function. FASEB J. 2002; 16: 123–125.
- Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress and antioxidants: a review. Journal of Biochemical and Molecular Toxicology. 2003; 17: 24–38.
- Coman C, Rugina OD, Socaciu C. Plants and Natural Compounds with Antidiabetic Action. Not Bot Horti Agrobo. 2012; 40: 314-325.
- Soltani M, Parivar K, Baharara J, Kerachian MA, Javad Asili J. Hemolytic and cytotoxic properties of saponin purified from Holothuria leucospilota sea cucumber. Reports of Biochemistry & Molecular Biology. 2014; 3: 43-50.
- Ridzwan BH. Sea Cucumbers A Malaysian Heritage, 1st Ed. Research Management Centre of International Islamic University Malaysia (IIUM): Kuala Lumpur Wilayah Persekutuan, Malaysia. 2010.
- Ming S. Investigation on Component and Pharmacology of Sea Cucumber. Chin. Tradit. Pat. Med. 2001.
- Althunibat OY, Ridzwan BH, Taher M, Jamaludin MD, Ikeda MA, Zali BI. In vitro antioxidant and anti proliferative activities of three Malaysian sea cucumber species. Eur. J. Sci. Res. 2009; 37: 376–387.
- Van Dyck S, Gerbaux P, Flammang P. Elucidation of molecular diversity and body distribution of saponins in the sea cucumber Holothuria forskali (Echinodermata) by mass spectrometry. Comp. Biochem. Physiol. B. 2009; 152: 124–134.
- Sottorff I, Aballay A, Hernández V, Roa L, Muñoz LX, Silva M. Characterization of bioactive molecules isolated from sea cucumber Athyonidium chilensis. Rev biol mar oceanogr. 2013; 48: 23-35.
- Fu X, Wen M, Han X, Yanagita T, Xue Y, Wang J, Xue C, Wang Y. Effect and potential mechanism of action of sea cucumber saponins on postprandial blood glucose in mice. Bioscience, Biotechnology and Biochemistry. 2016; 80: 1081-1087.
- Sharma OP, Kumar N, Singh B, Bhat TK. An improved method for thin layer chromatographic analysis of saponins. Food Chem. 2012; 132: 671-674.
- Karimi E, Jaafar HZE, Ahmad S. Phytochemical analysis and antimicrobial activities of methanolic extracts of leaf, stem and root from different varieties of Labisia pumila Benth. Molecules. 2011; 16: 4438-4450.
- Augustin JM, Kuzina V, Andersen S.B and Bak S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry. 2011; 72: 435–457.
- Francis G, Kerem Z, Makkar HP, Becker K. The biological action of saponins in animal systems: a review. Br J Nutr. 2002; 88: 587–605.
- Alonso–Vale MI, Peres SB, Vernochet C, Farmer SR, Lima FB. Adipocyte differentiation is inhibited by melatonin through the regulation of C/EBP beta transcriptional activity. J Pineal Res. 2009; 47: 221–227.
- Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc, Itani SI, et al. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-Co A carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA. 2002; 99: 16309-16313.
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S and et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002; 8: 1288- 1295.
- Mac Dougald OA, Burant CF. The rapidly expanding family of adipokines. Cell Metab. 2007; 6: 159-161.
- Chang J, Li Y, Huang Y, Lam KS, Hoo RL, Wong WT, Cheng KK and et al. Adiponectin prevents diabetic premature senescence of endothelial progenitor cells and promotes endothelial repair by suppressing the p38 MAP kinase/p16INK4A signaling pathway. Diabetes. 2010; 59: 2949–2959.
- Schalkwijk CG, Chaturvedi N, Schram MT. Adiponectin is inversely associated with renal function in type 1 diabetic patients. J Clin Endocrinol Metab. 2006; 91: 129–135.
- El Barky AR, Hussein SA, Alm-Eldeen AA, Hafez YA, Mohamed TM. Anti-diabetic activity of Holothuria thomasi saponin. Biomedicine & Pharmacotherapy. 2016; 84: 1472–1487.
- Leth H, Andersen KK, Frystyk J, Tarnow L, Rossing P, Paeving HH. Elevated levels of high-molecular weight adiponectin in type 1 diabetes. J Clin Endocrinol Metab. 2008; 93: 3186-3191.
- Oh DK, Ciaraldi T, Henry RR. Adiponectin in health and disease. Diabetes Obes Metab. 2007; 9: 282-289.
- Lindström T, Frystyk J, Hedman CA, Flyvbjerg A, Arnqvist HJ. Elevated circulating adiponectin in type 1 diabetes is associated with long diabetes duration. Clin. Endocrinol. 2006; 65: 776–782.
- Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes. 2003; 52: 1355–1363.
- Perez-Matute P, Zulet MA, Martinez JA. Reactive species and diabetes: counteracting oxidative stress to improve health. Current Opinion in Pharmacology. 2009; 9: 771–779.
- Faloon PW, Chou DHC, Forbeck EM, Walpita D, Morgan B, Buhrlage S and et al. Identification of Small Molecule Inhibitors that Suppress Cytokine-Induced Apoptosis in Human Pancreatic Islet Cells. Probe Reports from the NIH Molecular Libraries Program [Internet]. Bethesda (MD): NCBI (US); 2011.
- Kristiansen OP, Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005; 54: 114-124.
- El-Hadidy WF, Mohamed AR, Mannaa HF. Possible protective effect of procainamide as an epigenetic modifying agent in experimentally induced type 2 diabetes mellitus in rats, Alexandria J. Med. 2015; 51: 65–71.
- Chun FW, Xiu LB, Jing YY, Jia YZ, Ying XD, Jin HW and et al. Differential effects of ginsenoides on NO and TNF-a production by LPS-activatedN9 microglia. Int. Immunopharmacol. 2007; 7: 313–320.
- Srihardyastutie A, Soeatmadji DW, Fatchiyah and Aulanni’am. Relation of Elevated Serum Lipase to Indonesian Type 2 Diabetes Mellitus Progression. Biomedical Research. 2015; 26: 293-298.
- Burski K, Ueland T, Maciejewski R. Serum amylase activity disorders in the course of experimental diabetes in rabbits. Vet. Med.-Czech. 2004; 49: 197– 200.
- Burski K, Ueland T, Maciejewski R. Serum amylase activity disorders in the course of experimental diabetes in rabbits. Vet. Med.-Czech. 2004; 49: 197– 200.
- Kluge R, Scherneck S, Schürmann A, Joost HG. Pathophysiology and genetics of obesity and diabetes in the New Zealand obese mouse: A model of the human metabolic syndrome. Methods Mol Siol. 2012; 933: 59-73.
- Mossner J, Logsdon CD, Goldfine ID, Williams JA. Regulation of pancreatic acinar cell insulin receptors by insulin. Am J Physiol. 1984; 247: 155-160.
- Yadav UC, Moorthy K, Baquern Z. Combined treatment of sodium orthovanadate and Momordica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats. Mol Cell Biochem. 2005; 268: 111–120.
- El-Kaissi S, Sherbeeni S. Pharmacological management of type 2 diabetes mellitus: an update Curr Diabetes, Rev. 2011; 7: 392–405.
- Sudha P, Zinjarde SS, Bhargava SY, Kumar AR. Potent α-amylase inhibitory activity of Indian ayurvedic medicinal plants. BMC Complement Altern Med. 2011; 11: 5.
- Pulok KM, Kuntal M, Kakali M, Peter JH. Leads from Indian medicinal plants with hypoglycemic potentials. J Ethnopharmacol. 2006; 106: 1–28.
- McCue P, Shetty K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac J Clin Nut r. 2004; 13: 101-106.
- Kaur A, Verma N. Proteomics: A hallmark tool for identification of biomarker (LIPASE) in type I & II diabetes mellitus patients. Adv Appl Sci Res. 2012; 3: 1842–1847.
- Padalkar RK, Shinde AV, Patil SM. Lipid profile, serum malondialdehyde, superoxide dismutase in chronic kidney diseases and type 2 diabetes mellitus. Biomed Res. 2012; 23: 207–210.
- American Diabetes Association. Standards of medical care in diabetes-2013. Diabates Care. 2013; 36: 11–66.
- Malloy J, Gurney K, Shan K, Yan P, Chen S. Increased variability and abnormalities in pancreatic enzyme concentrations in otherwise asymptomatic subjects with type 2 diabetes. Diabetes Metab Syndr Obes. 2012; 5: 419–424.
- Aughsteen AA, Abu-Umair MS, Mahmoud SA. Biochemical analysis of serum pancreatic amylase and lipase enzymes in patients with type 1 and type 2 diabetes mellitus. Saudi Med J. 2005; 26: 73-77.
- Khotimchenko YS. The Nutritional value of holothurians. Russian Journal of Marine Biology. 2015; 41: 409–423.
- Hu XQ, Xu J, Xue Y and et al. Effects of bioactive components of sea cucumber on the serum, liver lipid profile and lipid absorption. Biosci, Biotechnol, Biochem. 2012; 76: 2214–2218.
- Taskinen MR. Diabetic dyslipidemia. Atherosclerosis Supplements. 2002; 3: 47-51.
- Erejuwa OO, Sulaiman SA, Ab Wahab MS. Honey- a novel antidiabetic agent. Int J Biol Sci. 2012; 8: 913-934.
- Mathe D. Dyslipidemia and diabetes: animal models. Diabetes Metab. 1995; 21: 106-111.
- Natarajan R, Nadler JL. Lipoxygenoses and lipid signaling in vascular cells in diabetes. Front Biosci. 2003; 8: 783-795.
- VanTol A. Hypertriglyceridemia in diabetic rat, defective removal of serum VLD. Atherosclerosis. 1977; 26: 117–128.
- Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007; 68: 72-82.
- Hu X, Wang Y, Wang J, Xue Y, Li Z, Nagao K, Yanagita T, Xue C. Dietary saponins of sea cucumber alleviate orotic acid-induced fatty liver in rats via PPAR alpha and SREBP-1c signaling. Lipids in Health and Disease. 2010; 9: 25.
- Rotimi SO, Omotosho OE, Rotimi OA. Persistence of acidosis in alloxan induced diabetic rats treated with the juice of Asystasia gangetica leaves. Phcog. Mag. 2011; 7: 25-30.
- Olalekan EO, Omotuyi, Olaposi I, Kamdem, Paul J and et al. Saponin as regulator of biofuel: implication for ethno botanical management of diabetes. J. Physiobioche. 2014; 70: 555-567.
- Zhang J, Zhao S, Yin P, Yan L, Han J, Shi L and et al. α-Glucosidase Inhibitory Activity of Polyphenols from the Burs of Castanea mollissima Blume. Molecules. 2014; 19: 8373-8386
- Furman BL. Streptozotocin-Induced Diabetic Models in Mice and Rats. Pharmacology. 2015; 70: 1-20.
- Zhang F, Ye C, Li G, Ding W, Zhou W and et al. The rat model of type 2 diabetic mellitus and its glycometabolism characters. Exp Anim. 2003; 52: 401-407.
- Malini P, Kanchana G, Rajadurai M. Antibiabetic efficacy of allagic acid instreptozotocin induced diabetes mellitus in albino wistar rats. Asian J Pharm Clin Res. 2011; 4: 124-128.
- Ventura-Sobrevilla J, Boone-Villa VD, Aguilar CN, Román-Ramos R, Vega-Ávila E, Campos-Sepúlveda E, Alarcón-Aguilar F. Effect of Varying dose and administration of Streptozotocin on blood sugar in male CD1 mice. Proc West Pharmacol Soc. 2011; 54: 5–9.