
Research Article
Austin Pancreat Disord. 2019; 3(1): 1013.
Antiobesity and Inhibitory Pancreatic Lipase Effects of Bioactive Compounds of Pistacia atlantica Roots Extract
Ben Hmed M1, Rigane G3, Ben Salem R2, Zouari N4 and Cherif S5*
1Reasearch Unit of Macromolecular Biochemistry and Genetic, Faculty of Sciences of Gafsa, University of Gafsa, Tunisia
2Laboratory of Organic Chemistry LR17ES08, Sciences Faculty of Sfax, University of Sfax, Tunisia
3Department of Chemistry-Physics, Sciences and Technology Faculty, Sidi Bouzid, University of Kairouan, Tunisia
4Higher Institute of Applied Biology ISBAM Medenine 4119, University of Gabes, Tunisia
5Department of Biological Engineering, Unité de Biotechnologie des Algues, UR 17 ES 42, University of Sfax, Sfax, Tunisia
*Corresponding author: Cherif S, Department of Biological Engineering, Unité de Biotechnologie des Algues, UR 17 ES 42, University of Sfax, Sfax, Tunisia
Received: November 25, 2019; Accepted: December 24, 2019; Published: December 31, 2019
Abstract
The identification and isolation of bioactive compounds are of great interest in the drug delivery field. We describe here, the identification of bioactive compounds, of aqueous extract obtained from Pistacia atlantica roots (PARE). Applying Liquid chromatography Electrospray Ionization-Tandem/mass spectrometry (LC-ESI-MS) to determine the composition of PARE, twelve metabolites were recognized. The main compounds were quinic acid (960 μg/g dry weight dw) and protocatechuic acid (452 μg/g dw). The inhibitory effect of PARE on porcine pancreatic lipase was also evaluated, and our results revealed that this extract markedly reduced lipase activity. In fact, the highest lipase activity inhibitory (100%) was observed at a concentration of 1 mg/ml after 30 min of incubation.
Keywords: LC-ESI-MS; Pistacia atlantica; roots; aqueous extract; bioactive compounds; pancreatic lipase inhibition
Introduction
Phenolic compounds constitute one of the most numerous and ubiquitous groups, of the secondary metabolites. Epidemiological, clinical, and nutritional studies strongly support the evidence that plant phenolic compounds enhance human health by reducing risks of various diseases, including cancers, cardiovascular diseases, degenerative diseases, and metabolic disorders. Many bioactive principles from plants have been reported to exhibit strong role in the prevention of obesity [1]. Moreover, some literatures have also reported that phenolic-rich extracts from various plant materials exhibited good inhibitory effects on digestive enzymes, e.g., a-amylase, a- glucosidase and pancreatic lipase [2-5]. The genus Pistacia (Anacardiaceae) consists of at least 11 species. Plants of this genus are primarily distributed in the Northern hemisphere and include both evergreen and deciduous species Pistacia atlantica is one of the most widely distributed wild species, called ‘Elbutom’ in Tunisia, and is the most characteristic plant species of the arid and semi-arid regions of the country [6]. Pistacia atlantica is believed to have several therapeutic properties. Scientific findings also revealed the wide pharmacological activities such as antioxidant, antimicrobial and antihyperglycemic activities from various parts of this species [7- 8]. Nevertheless, limited research has been conducted on the antiobesity activities of this plant. Hence, the aim of this study was to evaluate for the first time, the powerful inhibitory effect of the roots aqueous extract of this plant against porcine pancreatic lipase.
Materials and Methods
Plant material and preparation of extract
The roots of Pistacia atlantica were sampled from Gafsa, south western Tunisia, in January 2017 and deposited at the herbarium in the Faculty of Sciences, University of Gafsa, Tunisia. 10 g of Roots were washed with distilled water and dried at room temperature (approximately 20% relative humidity and 24% temperature) for 48h. Then, the samples were ground to fine powder using a blender (Moulinex, France) and extracted for 24h with water for three times at room temperature with magnetic stirring. After filtration using Whatman number 1 filter paper, the mixture was centrifuged for 10 min at 4500 g. The supernatant was lyophilized and stored at -20°C, until use.
LC-ESI-MS analysis
The extract (20 mg/ml) was filtered through a 0.45 μm pore size membrane (Merck, Darmstadt, Germany) before being injected into the ultra-fast liquid chromatography system. The liquid chromatography Electrospray Ionization Tandem Mass Spectrometry (LC-ESI-MS) analysis was done using LCMS-2020 quadrupole mass spectrometer (Shimadzu, Kyoto, Japan), equipped with an Electrospray Ionization Source (ESI), and operated in negative ionization mode. The mass spectrometer was coupled in-line with an ultra-fast liquid chromatography system consisting of a LC- 20 CE XR binary pump system, SIL-20AC XR auto sampler, CTO- 20AC column and DGU-20A 3 R degasser (Shimadzu). An Aquasil C18 column (Thermo Electron, Dreieich, Germany) (150mm×3 mm, 3 μm) preceded by an Aquasil C18 guard column (10 mm × 3 mm, 3 μm, Thermo Electron) were used for the analysis. The mobile phase was A (0.1% formic acid in H2O, v/v) and B (0.1% formic acid in methanol, v/v) with a linear gradient elution: 0–45 min, 10–100% B; 45–55 min, 100% B. Re-equilibration duration was 5 min between individual runs. The column temperature was maintained at 40°C, the mobile phase flow rate was 0.4 ml/min and the injection volume was 5 μl. High-purity (›99%) nitrogen was used as a nebulizer and an auxiliary gas. The spectra were monitored in Selected-Ion Monitoring (SIM) mode and processed using Shimadzu Lab Solutions LC-MS software. The mass spectrometer was operated in negative ion mode with a capillary voltage of -3.5 V, a dry gas flow rate of 12 l/min, a nebulizing gas flow rate of 1.5 l/min, a block source temperature of 400°C, a dissolving line temperature of 250°C, a voltage detector of 1.2 V and the full scan spectra from 50 to 2000 m/z. The identification of phenolic compounds was done by comparing the retention times and the mass spectra witht hose of authentic standards. The chemical standards such as: quinic acid, gallic acid, protocatechuic acid, chlorogenic acid, caffeic acid, syringic acid, p-coumaric acid, trans ferulic acid, o-coumaric acid, trans-cinnamic acid, 4-O-caffeoylquinic acid, 1,3-di-o-caffeoylquinic acid, 3,4-di-o-caffeoylquinic acid, 4,5-dio- caffeoylquinic acid, rosmarinic acid, salvianolic acid, catechin (+), epicatechin, acacetin, apigenin-7-O-glucoside, apigenin, cirsilineol, cirsiliol, hyperoside (quercetin-3-O-galactoside), luteolin-7-Oglucoside, luteolin, naringenin, naringin, quercitrin (quercetin-3-Orhamonoside), quercetin, rutin and silymarin) were purchased from Sigma Chemical Co. (St Louis, MO, USA) and with purity higher than ≥99.0%).
Enzyme
Porcine Pancreatic Lipase (PPL) was purchased from Sigma Chemical (St. Louis, USA).
Measurement of pancreatic lipase activity
Lipase activity was determined spectrophotometrically using p-nitrophenyl palmitate (p-NPP) as substrate according to the method of Nawani et al. with some modifications. The reaction mixture containing 0.3 mL of 0.05 M phosphate buffer (pH 8.0), 0.1 mL of 0.8 mM p-NPP and 0.1 mL of lipase was incubated at 37°C for 10 min. The reaction was then terminated by adding 1 mL of ethanol. A control was run simultaneously, which contained the same contents but the reaction was terminated prior to the addition of enzyme. Absorbance of the resulting yellow colored product was measured at 410 nm in a spectrophotometer. One International Unit (IU) of lipase activity was defined as the amount of enzyme catalyzing the release of 1 μmol of p-nitrophenol per min from p-NPP under the standard assay conditions.
Measurement of inhibitory effects of PARE on porcine pancreatic lipase
Three methods were used to determine the inhibitory effect of aqueous extract depending on the order of addition of lipase, substrate and inhibitor.
Method A: Lipase-inhibitor preincubation. This method was used to test the possible reaction between lipase and inhibitor in the absence of substrate. Lipase-inhibitor pre-incubations were performed at 25°C, different times and at various inhibitor extract amounts (aI). The final enzyme concentration in the incubation medium was 1 μM. The residual lipase activity was measured spectrophotometrically using p-nitrophenyl palmitate (p-NPP) as substrate. The amount of the inhibitor fraction (aI50) and the half-inactivation time (t1/2), corresponding to 50% of residual enzyme activity, were determined. In each case, control experiments were performed in the absence of the inhibitor fraction but with the same volume of DMSO. It is worth noting that DMSO at a final volume concentration less than 10% has no effect on the enzyme activity.
Method B: In this case, the inhibitor was added during lipolysis. This method was designed to test the possible inactivation reaction in the presence of substrate.
Method C or A’: The experimental conditions were run as in method A with the addition of NaTDC at 4 mM final concentration to create a micellar solution containing the inhibitory molecules.
Statistical analysis
Experimental results were given as mean ± standard deviation (mean ± SD) of three separate experiments. Statistical analysis was conducted using by one-way ANOVA, followed by Turkey test. Differences at p ‹ 0.05 were considered to be significant.
Results and Discussion
Chemical composition of Pistacia atlantica roots aqueous extract
Pistacia atlantica roots aqueous extract was analyzed by (LC-ESIMS) using a LCMS-2020 quadrupole mass spectrometer (Shimadzu, Kyoto, Japan) equipped with an Electrospray Ionization Source (ESI) and operated in negative ionization mode. Twelve components were identified (Data not shown). The major phenolic identified acids were quinic acid. Its concentration was noticeable and is about 960 μg/g extract. The results showed also the presence of protocatechuic acid, gallic acid, and catechin. Their concentrations were 452, 349 and 81 μg/g extract, respectively. As yet, there is no reports about the identification of polyphenolic compounds in P. atlantica root extract. Ben amar et al., have demonstrated that quinic acid and gallic acid were present in P. atlantica leaves aqueous extract [10]. More recently, Ahmed et al., have shown that quinic acid, gallic acid and other compounds were present in P. atlantica leaves extract [11]. In fact, these compounds exerted hypoglycemic effect by inhibiting a-amylase. Moreover, several reports have demonstrated the pharmacological activities of protocatechuic acid including anti-inflammatory as well as anti-hyperglycemic and antimicrobial activities. This compound was reported also to be efficient by exerting an anti-proliferative effect in cancerous tissues [12]. Furthermore, the presence of other phenolic acid and flavonoids such as synergic acid, p-coumaric acid, epicatechin and cirsiliol were also identified. These compounds display interesting biological properties, such as anticarcinogenic, anti-inflammatory, antimicrobial, antioxidant, antiageing, antiallergic, antidiabetic, neuroprotective, radioprotective, and hepatoprotective [13-14]. Similarly, Rezaie et al have reported the presence of gallic acid, rutin, quercetin and its derivates in the hulls of P. atlantica using HPLC-MS and NMR techniques along with many other compounds not identified [15].
Inhibitory effect of PARE against lipase of medical interest
Inhibiting lipases has potential applications in the field of medicine. The inhibitory activity of PARE was evaluated against Porcine Pancreatic Lipase (PPL) using three methods. Linear kinetics corresponding to the FFAs (μmol) released vs. time (min) was obtained in the presence and absence of the inhibitor fraction. A dose-dependent effect was observed upon increasing the amount of PARE on PPL (Figure 1). At an aI value of only 50 μg, PPL activity was totally abolished after a 30 min incubation period (Figure 1A), while PPL was strongly inactivated (95%) at an aI of up 200 μg when using method B (Figure 1B).To mimic the in vivo conditions, PARE extract were incubated with PPL and with a supramicellar concentration (4 mM) of bile salts (NaTDC). Our results show that, as compared to the assay without NaTDC, a similar inhibition profile was obtained on the presence of 4 mM of NaTDC (Figure 1C). The aI50 values were found to be 10 μg, 65 μg and 60 μg for inhibition method A, B, and C, respectively (Table 1). This result could be explained by the fact of the richness of the extracts on bioactive molecules, which act in synergy or the molecule responsible for the inhibition is polar and cannot reach the interface when the enzyme is adsorbed. This case was observed in the case of the Tetrahydrolipstatin (THL). The increase in the inhibitory efficacy of THL by bile salts over their Critical Micellar Concentration (CMC) was reported in the case of pancreatic lipases in human as well for HGL and lipoprotein lipase [16-17]. As it was reported, there is a difference in the association of the enzyme to the surface. Some compounds, such as THL and diethyl-p-nitro phenyl phosphate (E600), covalently modify the lipase molecule by reaction with the catalytic serine [18]. In this latter case, Rouard et al. [19], showed that E600 present in mixed micelles of bile salts irreversibly inactivates porcine pancreatic lipase, whereas aqueous solution of this organo-phosphorous compound does not. Similar effects were reported by Desnuelle et al. [20], in presence or in absence of emulsified E600 with gum Arabic. It appears thus that the availability and/or the organization of these inhibitors at an interface is one of the most decisive factors on which their efficacy depends.
Methods
aI 50
t ½ (min)
A
10
1
B
65
C
60
1,2
Table 1: aI50> and t1/2 values of PARE.
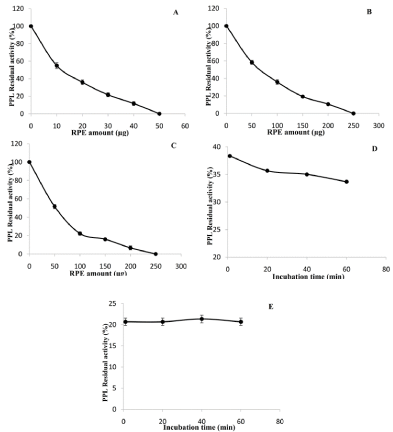
Figure 1: Evaluation of the inhibitory effect of PARE against PPL using
Method A (A), method B (B) and method C (C). The residual activities of PPL
were measured as a function of the incubation time at a constant amount of
PARE using method A (D) and method C (E).
The influence of the incubation time on the level of inhibition of PPL using method A and C by RPE was further investigated (Figure 1D, 1E).For both methods (A and C), the residual activity decreased rapidly and reached a plateau value after approximately 30 min of incubation. From these inhibition curves, the values of the half-inactivation time (t1/2) were then determined and found to be 1 and 1.2 min for method A and C, respectively (Table 1). Such values reflect an extremely high rate of inhibition of PPL by PARE.
Conclusion
In the present work, the identification of phenolic compounds, and the lipase inhibitory activity of P. atlantica roots aqueous extract was examined for the first time. Our result showed that PARE displayed a noticeable lipase inhibitory activity against porcine pancreatic lipase. These finding suggest that P. atlantica root extract might have the potential to serve as a source of a potential antiobesity agent. However, further studies are needed to identify the compound responsible for the ant obesity activity and to evaluate the effect of the extract in in vivo model.
Acknowledgements
This work was funded by the Tunisian Ministry of Higher Education and Scientific Research.
References
- Yamagata K, Tagami M, Yamori Y. Dietary polyphenols regulate endothelial function and prevent cardiovascular disease. Nutrition. 2015; 31: 28-37.
- Yuan Y, Zhang J, Fan J, Clark J, Shen P, Li Y, Zhang C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on a-amylase, a-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018; 113: 288-297.
- Quettier-deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Cazin M. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flou. J. Ethnopharmacol. 2000; 72: 35-42.
- Zhang C, Ma Y, Gao F, Zhao Y, Cai S, Pang M. The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis Mill. Fruits and their pancreatic lipase inhibitory activities with molecular docking analysis. J. Funct. Foods. 2018; 40: 729-735.
- Camargo A C, Regitano-D’arce M A B, Biasoto A C T, Shahidi F. Enzyme-assisted extraction of phenolics from winemaking by-products: Antioxidant potential and inhibition of alpha-glucosidase and lipase activities. Food Chem. 2016: 212: 395-402.
- El Zerey-Belaskri A, Benhassaini H. Morphological leaf variability in natural populations of Pistacia atlantica Desf. subsp. atlantica along climatic gradient: new features to update Pistacia atlantica subsp. Atlantica key. Int J Biometeorol. 2016; 60: 577-589.
- Ben Ahmed Z, Yousfi M, Viaene J, Dejaegher B, Demeyer K, Mangelings D, Vander Heyden Y. Antioxidant activities of Pistacia atlantica extracts modeled as a function of chromatographic fingerprints in order to identify antioxidant markers. Microchem J. 2016; 128: 208-217.
- Ben hammou N, Bekkara F A, Panovska T K. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. Afr J Pharm Pharmacol. 2008: 2: 22-28.
- Nawani J, Khurana J. Mol Cell Biochem. 2006; 290: 17-22.
- Benamar H, Marouf A, Bennaceur M. Phytochemical composition, antioxidant and acetylcholinesterase inhibitory activities of aqueous extract and fractions of Pistacia atlantica subsp. atlantica from Algeria. Journal of Herbs, Spices & Medicinal Plants. 2018; 24: 229-244.
- Ben Ahmed Z, Yousfi M, Viaene J, Dejaegher B, Demeyer K, Mangelings D, Vander Heyden Y. Potentially antidiabetic and antihypertensive compounds identified from Pistacia atlantica leaf extracts by LC fingerprinting. J. Pharm. Biomed. Anal. 2018; 149: 547-556.
- Semaming Y, Pannengpetch P, Chattipakorn N. Pharmacological properties of protocatechuic acid and its potential roles as complementary medicine. Evid. Based Complement. Alternat. Med. 2015; 1-11.
- Kang J, Kim E, Kim W, Seong K M, Youn H, Kim J W, Kim J, Youn B. Rhamnetin and cirsiliol induce radiosensitization and inhibition of epithelial-mesenchymal 20 transition (EMT) by miR-34a-mediated suppression of Notch-1 expression in non-small cell lung cancer cell lines. Journal of Biological Chemistry. 2013; 288: 27343-27357.
- Lesjak M, Beara I, Simin N, Pintac D, Majkic T, Bekvalac K, Mimica-Dukic N, Lesjak M. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. Journal of Functional Foods. 2018; 40: 68-75.
- Rezaie M, Farhoush R, Pham N, Quinn R J, Iranshahi M. Dereplication of antioxidant compounds in Bene (Pistacia atlantica subsp. mutica) hull using a multiplex approach of HPLC-DAD, LC-MS and 1H NMR techniques. J. Pharm. Biomed. Anal. 2016; 117: 352-362.
- LüthiPeng Q, Winkler F K. Large spectral changes accompany the conformational transition of human pancreatic lipase induced by acylation with the inhibitor tetrahydrolipstatin. Eur. J. Biochem. 1992; 205: 383-390.
- Lookene A, Skottova N, Olivecrona G. Interactions of lipoprotein lipase with the active-site inhibitor tetrahydrolipstatin (Orlistat). Eur. J. Biochem. 1994; 222: 395-403.
- Hadvàry P, Lengsfeld H, Wolfer H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipsatin. Biochem. 1988; 256: 357-361.
- Rouard M, Sari H, Nurit S, Entressangles B, Desnuelle P. Inactivation of pancreatic lipase by mixed micelles of diethyl p- nitrophenyl phosphate and Bile salts. Biochim Biophys. Acta. 1978; 530: 227-235.
- Desnuelle P, Sarda L, Ailhaud G. Inhibition of pancreatic lipase by the diéthyl-p-nitrophényl phosphate in emulsion. Biochim. Biophys. Acta. 1960; 37: 570-571.