Editorial
In the modern era of multidisciplinary medicine, towards a personalized approach to the patients, the surgical pathologists are called to respond to peculiar questions as well as to address important issues. In the complex landscape of surgical pathology, made by routine gross sampling, frozen-section analysis, histopathologic diagnosis and molecular characterization of cancer, the necessity of a standardization of the methods becomes mandatory. And not only the methods need standardization, but even the results. Indeed, in every aspect of surgical pathology, it is now fundamental fixing standardized parameters and establishing cut-offs and thresholds. Starting from gross approach, the sampling of particular organs or tissues has to follow strict guidelines. For example, the sentinel lymph node of a patient with breast carcinoma, if it is larger than 5 millimeters, has to be cut with transversal section of 3 millimeters. In the evaluation of a surgical specimen for tumor, furthermore, the pathologist has to document with appropriate sections not only the presence of tumor, but even the status of surgical margins. In a pancreaticoduodenectomy for pancreatic ductal adenocarcinoma, one of the most difficult surgical specimens in the process of gross sampling, the pathologist has to separate the neck margin, the biliary margin, and, ideally marking with black ink, the retroperitoneal margin. This method of sampling is fundamental to write a correct and exhaustive final pathology report. And for the same reason, also the lymph nodes have to be correctly isolated from the adipose tissue and included for histologic exam. Since the information in the final pathology report is essential to address the therapy, it needs a clear, reliable and reproducible standardization. In the diagnostic routine, moreover, to assess the proliferative index of breast carcinoma, the pathologists all around the world tend to consider the particular staining pattern of Ki-67 (MIB1) in immunohistochemistry: on the basis of this pattern and of already well-established thresholds, this tumor can be classified as a low or a high-rate proliferative tumor. However, there are many immunohistochemical analyses that still need, nowadays, standardization. Another example in pathology is given by the standardization process of a new antibody, or of an antibody for new aims, in immunohistochemistry [1,2]: finding thresholds and cut-offs is really essential. About immunohistochemistry, moreover, also the fixation level has to be clearly standardized: a not-well fixed specimen can be misinterpreted as negative or focally positive (Figure 1). Nowadays, however, thanks to the exponential growth of biological knowledge and to the massive production of genetic/mutation data (DNA sequencing of cancer, issue of tumor heterogeneity), there are too much values and parameters that have to be considered to perform the so-called “next-generation histopathologic diagnosis” [3], but there are not precise thresholds or cut-offs for every new parameter. In a big lesion, how many are the sections that the pathologist has to cut? Is there a minimum? Is there a maximum? And how many are the sections in which the pathologist has to perform the immunohistochemical analysis to consider it representative of the entire tumor? And how many are the sections in which the pathologists have to investigate particular gene mutations? And which is the role played by the issue of genetic heterogeneity in surgical and molecular pathology? In this complex situation, in which the consensus conference or meeting are still very important, the perfect tool for modern surgical pathologists is certainly represented by meta-analysis. With this statistical method, we will be able not only to scientifically approach to particular issues but also to look for the most important parameters in specific cancers and to establish the thresholds that can guide the pathologists during the diagnostic activity. One of the most important qualities of the meta-analytic approach, in fact, is that it allows to summarize the findings coming from several studies, overcoming the limitations of the small sample sizes or of the limited number of cases, unfortunately present in many studies. The obtained results, furthermore, can be very heterogeneous, but in this situation the meta-regression analysis can investigate this kind of issue giving the answer of such heterogeneity in the results, often highlighting problems or solutions. The meta-analysis can be thus applied to two main aspects of surgical pathology: i) the prognostic impact of documenting the mutation status of particular genes in cancer [4]; ii) the prognostic role of macro- or microscopic features of cancers [5-8]. Many other aspects can be studied with meta-analysis but, for pathologists, in our opinion, these two ways are the most important in this time.
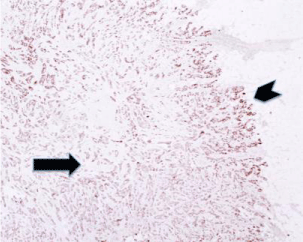
Figure 1: Sub-optimal fixation in formalin can give false-negative results in
immunohistochemistry. In this figure a delayed fixation gives a false negative
staining pattern in the center of the lesion (arrow) for Estrogen Receptor in
this Non-Special Type Ductal Breast Carcinoma (4X magnification). The
periphery of the lesion (chevron), better fixed, is positive; in the center the
formalin was not yet penetrated. The fixation represents a very classic aspect
that needs strict standardization in pathology, to avoid false results.
References
- Luchini C, Parcesepe P, Mafficini A, Nottegar A, Parolini C, Veronese N, et al. Specific expression patterns of epithelial to mesenchymal transition factors in gestational molar disease. Placenta. 2015; 36: 1318-1324.
- Luchini C, Parcesepe P, Nottegar A, Parolini C, Mafficini A, Remo A, et al. CD71 in gestational pathology: a versatile immunohistochemical marker with new possible applications. Appl Immunohistochem Mol Morphol. 2015.
- Luchini C, Capelli P, Fassan M, Simbolo M, Mafficini A, Pedica F, et al. Next-generation histopathologic diagnosis: a lesson from a hepatic carcinosarcoma. J Clin Oncol. 2014; 32: e63-66.
- Luchini C, Veronese N, Solmi M, Cho H, Kim JH, Chou A. Prognostic role and implications of mutation status of tumor suppressor gene ARID1A in cancer: a systematic review and meta-analysis. Oncotarget. 2015; 6: 39088-39097.
- Veronese N, Nottegar A, Pea A, Solmi M, Stubbs B, Capelli P, et al. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol. 2016; 27: 42-48.
- Veronese N, Luchini C, Nottegar A, Kaneko T, Sergi G, Manzato E, et al. Prognostic impact and implications of extra-nodal extension in lymphnode positive thyroid cancers: a systematic review and meta-analysis. J SurgOncol. 2015.
- Luchini C, Nottegar A, Solmi M, Sergi G, Manzato E, Capelli P, et al. Prognostic implications of extranodal extension in node-positive squamous cell carcinoma of the vulva: A systematic review and meta-analysis. Surg Oncol. 2015.
- Luchini C, Veronese N, Pea A, Sergi G, Manzato E, Nottegar A, et al. Extranodal extension in N1-adenocarcinoma of the pancreas and papilla of Vater: a systematic review and meta-analysis of its prognostic significance. Eur J Gastroenterol Hepatol. 2016; 28: 205-209.
