Abstract
Ovarian epithelial tumors with transitional cell features are well-recognized. They morphologically resemble urothelium or urothelial carcinomas of the urinary tract and are classified as transitional cell tumors of the ovary which currently includes Brenner tumors and transitional cell carcinomas of the ovary. Brenner tumors are further classified as benign, borderline and malignant subtypes. The clinical and biological features of transitional cell tumors of the ovary are not well defined and the histogenesis of Brenner tumors is still uncertain. We report here our Immunohistochemistry (IHC) findings of PAX8 and p63 on transitional cell tumors of the ovary, the cell lineage specific transcription factors for Mullerian and urothelial epithelial cells, respectively. We show here that PAX8 and p63 were mutually exclusively expressed in Brenner tumors (PAX8-/p63+) and transitional cell carcinomas of the ovary (PAX8+/p63-), underlying a cell lineage difference between these two tumors. We also report that transitional metaplasia is a common phenomenon of the fallopian tube epithelium in the fimbriae and may represent an additional innate differentiation potential of the fallopian tube epithelium. The metaplastic transition type epithelium closely resembles immature urothelial cells and Brenner tumors on IHC, but not transitional cell carcinoma of the ovary. We thus suggest an alternative histogenesis for Brenner tumors arising from fallopian tube epithelium derived ovarian inclusion cysts. The cell lineage difference between Benner tumors and transitional cell carcinomas justifies the use of PAX8 and p63 to accurately diagnose transitional cell tumors of the ovary and separation of these two tumors in future classification of epithelial ovarian tumors.
Keywords: Immunohistochemistry; Bilateral salpingo-oophorectomy
Introduction
Tumors histologically similar to benign urothelium or urothelial carcinomas of the urinary tract are well-recognized in the ovary and are classified as transitional cell tumor of the ovary, which currently includes Brenner tumors and transitional cell carcinomas of the ovary [1]. The Brenner tumors are further classified into benign, borderline and malignant types. The differential diagnosis of primary malignant transitional cell tumors of the ovary can be challenging because they are morphologically indistinguishable and have been traditionally dependent on the identification of benign or borderline components of Brenner tumors. When the benign or borderline components are present, the malignant transitional cell tumor is diagnosed as malignant Brenner tumor, otherwise, as transitional cell carcinomas of the ovary [2]. However, it is conceivable that the benign or borderline components may be overlooked or misinterpreted and thus leads to the misclassification of transitional cell tumors of the ovary.
Recent evidences have indicated that transitional cell carcinomas of the ovary may have distinct clinical and biological features. The transitional cell morphologies are reported more frequently seen in BRCA gene mutation related ovarian carcinomas [3]. Transitional cell carcinomas of the ovary were reported to have better response to the cisplatin-based chemotherapy and May response to the Poly- ADP Ribose Polymerase (PARP) inhibitors which are under clinical try for BRCA related ovarian carcinomas [4-6]. Thus, it is imperative to have an accurate diagnosis of transitional cell tumors of the ovary for proper chemotherapy and evaluation of the treatment effects. In addition, the histogenesis of Brenner tumors still is puzzling because of the unique histologic features [7].
In this study, we investigated the expression of PAX8, p63, as well as cytokeratin 5/6, 7, and 20 in transitional cell tumors of the ovary utilizing IHC. We also studied the expression of these markers in the recently recognized metaplastic transitional epithelium of the fallopian tube [8-10]. We report here that fallopian tube epithelium frequently undergoes transitional (or urothelial) cell differentiation in the fimbriae. The metaplastic transitional type epithelium closely resembles immature urothelium and Brenner tumors on IHC. We suggest that Brenner tumors arise in the ovary from the metaplastic transitional epithelium of the fallopian tube epithelium derived cysts and therefore have a unified histogenesis as other ovarian epithelial tumors, i.e., a direct Müllerian epithelial origin. We also suggest that a cell lineage-related approach with IHC including PAX8 and p63 is more reliable to distinguish transitional cell tumors of the ovary and hence will facilitate to further define these tumors.
Material and Methods
The tissues used in this study included 5 cases of fallopian tube with metaplastic transitional epithelium, 27 Brenner tumors (25 benign, 1 borderline, and 1 malignant), and 23 transitional cell carcinomas of the ovary. The H&E sections of tumor cases were reviewed and the corresponding diagnoses were confirmed. A Tissue Microarray (TMA) was assembled with three 0.6 mm cores of representative tissues from each benign Brenner tumor or ovarian transitional cell carcinoma for IHC study. The cases of metaplastic transitional epithelium were selected from the prophylactic sapingo-ovulectomy specimen for patients with BRCA 1 or 2 germline mutations or family history of breast and ovarian carcinomas. A TMA with 41 invasive urothelial carcinomas of the urinary bladder was used as control in this study.
IHC with PAX8 (Proteintech, Chicago, IL), p63 (Ventana, Tuscon, AZ) as well as CK5/6, CK7 and CK20 was performed in a Dako autostainer utilizing avidin-biotin peroxidase method after appropriate antigen retrieval according to the manufacture manual. Only distinct nuclear staining was considered to be positive for PAX8 and p63. The staining intensity was graded as negative, weak (1+), moderate (2+) and strong (3+).
Results
The metaplastic transitional epithelium of the fallopian tube is defined as areas of stratified epithelium composed of multiple layers of uniform epithelial cells with round or oval nuclei and nuclear grooves morphologically resembling the urothelium in the fallopian tube epithelium. We have reviewed the H&E sections of fallopian tubes from 30 RRSO specimens performed for patients with high risk of developing ovarian carcinomas either confirmed BRCA1 or BRCA2 germline mutation or strong family history of breast and ovarian carcinomas. The patients varied from 30 to 52 year old (an average of 41 years). All fallopian tubes were submitted entirely for histologic examination according to the recently proposed protocol which generated 8 to 12 sections (an average of 10 sections) for each case. Areas of transitional-like epithelium were found in five cases which were readily identified because of its stratification of multiple layers of epithelial cells distinct from the single layer of ciliated fallopian tube epithelium. The transitional type epithelium usually were present bilaterally (4/5), multifocal, and varied from 1 to 5 mm in width and 5 to 10 cell layers. The transitional type epithelium was found in the fimbriae either franked by or growing under the ciliated serous epithelium (5/5) as well as in the peritoneal-tubal junctions (2/5). The transitional-like areas are uniform and small and the cells in the superficial layers appeared to be larger and flatten, but there were no typical umbrella-like cells present (Figure 1). In addition to areas of transitional type epithelium, one with high grade serous intraepithelial carcinoma, one with endometrial metaplasia, and one with mucinous metaplasia (endocervical type) were also identified in these 30 cases (data not shown).
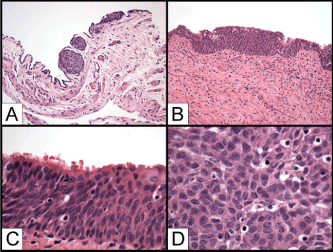
Figure 1: Transitional type Epithelium (TE) of the fallopian tube. A) TE in
the peritoneal-tubal conjunction (H&E, 50x); B) TE flanked by the ciliated
fallopian tube epithelium (H&E, 100x); C) TE under the ciliated fallopian tube
epithelium (H&E, 200x); D) TE with uniform oval nuclei and nuclear grooves
(H&E, 400x).
We next investigated the immunohistochemical features of the transitional-like epithelium in the fallopian tube utilizing IHC. We selected a short panel of antibodies against PAX8, p63, CK5/6, CK7, and CK20 because these proteins represent markers of cell lineages and differentiation. PAX8 is a nuclear protein for the Müllerian epithelial cell lineage marker and is expressed in the non-ciliated secretory (serous) cells of the fallopian tube epithelium, whereas p63 is the transcription factor required for the development of the stratified epithelium including squamous epithelium and urothelium. It is expressed in the immature urothelial cells in basal and intermediate layers or stem/progenitor cells in the urothelium. The cytokeratin expression has distinct pattern in the urothelium and reflects the differentiation stages of urothelial cells: CK7 in all urothelial cells (terminally differentiated or immature), CK5/6 in the maturing urothelial cells of the basal and intermediate urothelial layers, and CK20 in the terminally differentiated superficial umbrella cells. The transitional-like epithelium of the fallopian tube was positive for CK7, CK5/6, and p63; and negative for PAX8 and CK20. The CK7 staining was detected in all layers of the transitional-like epithelium of the fallopian tube epithelium; CK5/6 and p63 were detected in the basal and intermediate cells. The most superficial cells were usually negative for CK5/6 and p63 as well as for CK20 (Figure 2). The fallopian tube epithelium is diffusely positive for CK7 and focally positive for CK5/6; the interspersed non-ciliated serous cells were positive for PAX8; no p63 or CK20 staining was detected in the fallopian tube epithelium (data not shown).
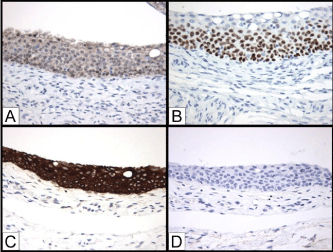
Figure 2: Immunohistochemistry (IHC) of transitional type epithelium of the
fallopian tube. A) PAX8; B) p63; C) CK7; D) CK20 (IHC, 20x).
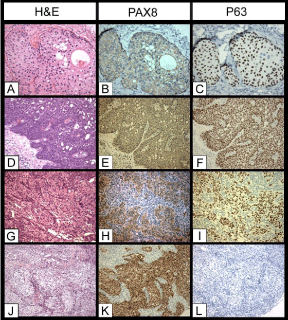
Figure 3: Expression of PAX8 and p63 in Brenner tumors and transitional cell
carcinomas of the ovary. A, B, C) Benign Brenner tumor, H&E, PAX8, and
p63 (20x), respectively; D, E, F) Borderline Brenner tumor, H&E, PAX8, and
p63 (20x), respectively; G, H, I) Malignant Brenner tumor, H&E, PAX8, p63,
respectively; J, K, L) Transitional cell carcinoma of the ovary, H&E, PAX8,
and p63, respectively.
We then investigated the expression of these markers in transitional cell tumors of the ovary. The 25 benign Brenner tumors were from 23 patients of 30 to 60 years old (average of 45 years old). Seven cases were incidental microscopic findings and were less than 1 cm in size. In these cases, the nests or microcysts with transitional-like epithelium were embedded in the dense fibrotic or stromal tissues. The others (18/25) had grossly identified tumors varied from 2 to 3.5 cm in size (average of 2.5 cm). Seven of these tumors were predominately solid, 6 were mainly cystic, and 5 were solid and cystic. Three of the cystic benign Brenner tumors were associated with benign mucinous cystadenomas. The tumors were located in the cortex, medulla or hilar areas and confined to the ovaries. The clinical and histologic features of the borderline and malignant Brenner tumors were described in previous publication [11]. For the transitional cell carcinomas, the transitional-like components either constituted the predominant portion of the ovarian tumors (>50%) or associated with other types of high grade ovarian carcinomas as a minor component. IHC stains showed the Brenner tumors (27/27), benign, borderline or malignant were positive for CK5/6, CK7, p63, negative for PAX8 and K20; all the transitional cell carcinomas of the ovary (23/23) were positive for CK7 and PAX8, negative for CK5/6, CK20, and p63. There was no co-expression of PAX8 and p63 detected in either Brenner tumors or transitional cell carcinomas of the ovary in this IHC study.
Discussion
The origin of ovarian carcinomas has been a matter of debate [12]. For decades, the ovarian surface epithelium has been considered as the tissue origin of ovarian carcinomas, especially for the high grade serous carcinomas. This was also known as “incessant ovulation theory” which attributed the ovulation-related ovarian surface epithelium damage and repair to ovarian carcinogenesis [13]. The incessant ovulation theory was mainly based on the observations of super ovulation increasing (in poultry hens) and suppression of ovulation decreasing (in women with multiple pregnancies or long-term use of contraceptives) ovarian carcinomas. However, the incessant ovulation theory was not able to adequately address some important issues in the ovarian carcinogenesis. For example, no convincing premalignant lesions have been identified on the ovarian surface epithelium despite of extensive investigation, which contradicts the stepwise progression of malignant epithelial neoplasms. In addition, there is significant difference between the purported tissue of origin and malignant tumors. The ovarian surface epithelium is in continuity with the pelvic peritoneum and is of mesothelium in nature whereas ovarian carcinomas are malignant epithelial tumors and different from the ovarian surface epithelium at histological, immunohistochemical and ultrastructural levels [14]. Nevertheless, the incessant ovulation theory was widely accepted and has dictated the efforts of treatment, prevention and basic research for ovarian carcinomas in last several decades.
It was until recently the theory was challenged and alternative theories gained increasing supports. The evidences emerged initially from patients with BRCA1 or BRCA2 germline mutations [15-17]. These patients have a significantly increased risk of developing high-grade serous carcinomas of the ovary. Bilateral Salpingo- Oophorectomy (BSO) is provided as a prophylactic and riskreducing measure for these patients. Such specimens provided unique opportunities to study the initiation of ovarian carcinomas. Histological examination of these specimens unexpectedly revealed that the long-sought premalignant lesions or occult (early) carcinomas were found primarily on the fimbria of fallopian tubes and were rarely seen on the ovarian surface epithelium. Subsequent studies demonstrated that such a phenomenon was not limited to the hereditary ovarian carcinomas and the fimbria of fallopian tubes of some sporadic ovarian carcinomas also harbored similar premalignant lesions [18]. In addition, gene expression profile [19,20] and cell lineage studies [21-24] also showed that papillary serous carcinomas of the ovary were more closely related to the fallopian tube epithelium instead of the ovarian surface epithelium. These findings strongly indicate that high-grade serous carcinomas of the ovary arise from the fimbria of fallopian tubes instead from the ovarian surface epithelium. The ovary is involved secondarily by tumor implantation or direct invasion from the fallopian tubes because of their close proximity. These tumors eventually “become” ovarian carcinomas because the ovary involvement is always extensive. These evidences have initiated the recent shift for ovarian carcinogenesis from the ovarian surface epithelium to the fallopian tube epithelium. The new model indicates that high-grade ovarian carcinomas has a direct Mullerian origin and derives from the Müllerian-derived fallopian tube epithelium in the fimbria of the fallopian tubes [7,25-28].
A direct Müllerian origin has been well-documented for other types of ovarian carcinomas such as endometrioid and clear cell adenocarcinomas of the ovary. Both tumors frequently arise from the preexisting endometriosis in the ovary or pelvis [29,30]. A related cell lineage with the fallopian tube epithelium was also demonstrated for low-grade serous ovarian epithelial tumors which may also arise from fallopian tube type epithelium [31]. However, it is intrigue whether the theory of direct Müllerian origin can be extended to all ovarian epithelial tumors, especially for those ovarian epithelial tumors with unique histological features. These tumors include Brenner tumors and gastrointestinal types of ovarian mucinous carcinomas, which contain neoplastic cells similar to epithelial cells in the urinary and gastrointestinal tract, respectively [7]. Here we propose that Brenner tumors may also directly arise from the Müllerian epithelium and may be related with the transitional cell metaplasia of the fallopian tube epithelium, an under-recognized cellular differentiation of the Müllerian epithelial at the distal end of the fallopian tubes.
Transitional cell metaplasia is a morphologic transformation of non-urothelial epithelium into urothelium-like epithelium, which is characterized by a stratified epithelium composed of uniform epithelial cells with moderate amount of cytoplasm, round or oval nuclei, and nuclear grooves. Transitional cell metaplasia has been identified in several types of epithelium, such as the respiratory epithelium, gastrointestinal epithelium, and mesothelium, which usually is the result of reactive epithelial changes to various chronic physiological or pathological stimuli. In the female genital tract, transitional cell metaplasia frequently occurs in the transformation zone of the uterine cervix but was believed very rare in the fallopian tube [32,33]. However, recent reports have shown that transitional cell metaplasia is also common in the fallopian tube and these findings were made possible because of the recent recognized significance of fallopian tubes in ovarian carcinogenesis [8-10]. When the fallopian tubes were examined entirely as for the specimens of RR-BSO, as much as 20% of these fallopian tubes contained areas of transitional type epithelium. The transitional cell metaplasia was reported to be in the plicae of the fimbriae or at the peritoneal-tubal junction of the serosal aspect of the fallopian tubes. In this study, we reviewed 30 cases of RR-BSO specimen from patients with BRCA1 or 2 germline mutation and the fallopian tubes were submitted entirely for histological examination. The transitional type epithelium was identified by the segments of stratified epithelium of variable length in the native single layer of ciliated epithelium of the fallopian tube and was more frequently seen in the tips or the lateral aspects of the fimbrial plicae (5/5) than in the peritoneal-tubal junction (2/5). The origin of transitional cell-type epithelium at the peritoneal-tubal junctions may be elusive because it could arise from either the mesothelium or fallopian tube epithelium; whereas the one in the fimbrial plicae most likely is the direct result of transitional cell metaplasia of the fallopian tube epithelium. The transitional cell metaplasia appeared to be the most common morphological changes in the RR-BSO specimens since only one (0.3%) of focal endometrioid epithelium, one (0.3%) endocervical type mucinous epithelium, and one of serous carcinoma in-situ were identified in the same cohort. It has been under-recognized mainly because of inadequate histological examination of the fallopian tubes in the past. Transitional cell metaplasia may represent one innate form of the differentiation potentials of the Müllerian epithelium, in addition to the well-recognized serous, glandular (endometrial), mucinous (endocervical) and clear cell morphologies [34].
The transitional type epithelium in the fallopian tube has not been fully characterized immunohistochemically. In this study, we investigated its immunohistochemical features with a panel of antibodies including for PAX8, p63, CK5/6, CK7, and CK20. PAX8 and p63 are the cell lineage specific transcription factors essential for the development of Müllerian epithelium [35] and urothelium [36], respectively. PAX8 is expressed in the Müllerian derived normal epithelium or neoplastic tissues and has been used for Müllerian cell lineage marker; p63 is expressed in the stem/progenitor cells in the urothelium and basal and intermediate urothelial cells and is the key regulator for the differentiation of urothelial cell lineage [37]. We have shown the transitional type epithelium in the fallopian tube was diffusely positive for p63, CK5/6, CK7, negative for PAX8 and CK20, resembling the immature urothelial cells in the urothelium. Findings of gain of p63 and loss of PAX8 expression in the transitional type epithelium in the fallopian tube epithelium further underlie the importance of these two transcription factors in the differentiation and maintenance of Mullerian or urothelial cell lineage features.
Brenner tumors are not only morphologically and but also immunohistochemically similar to urothelial neoplasms of the urinary tract and are significantly different from other common ovarian epithelial tumors [1,38,39]. The unique features have made the histogenesis of Brenner tumors even more intrigue. Brenner tumors were purported arising from the ovarian surface epithelium, Walthard nests, or even sex-cord tissue. In this study, we have shown that Brenner tumors closely resemble the metaplastic transitional type epithelium of the fallopian tube with characteristic expression of p63, CK5/6, CK7 [40-42], and absence of PAX8 expression. This leads us to propose an alternative pathway for Brenner tumor histogenesis and we suggest that Brenner tumors arise from the transitional type epithelium of the fallopian tube, especially from those fallopian tube epithelium-derived cysts in the ovarian parenchyma [43]. It was reported recently that as much as 70% of ovarian inclusion cysts were lined by fallopian tube type epithelium and derived from the fallopian tube. The fallopian tube epithelium in the ovarian inclusion cysts may still retain the potential of transitional cell metaplasia and give rise to Brenner tumors. The scenario of ovarian cyst origin is consistent with the distribution of Brenner tumors in the ovary. Other secondary Müllerian structures such as endometriosis, endosalpingiosis, and rete ovarii in the ovary conceivably can undergo transitional cell metaplasia and may contribute to the development of Brenner tumors as well.
Transitional cell carcinomas of the ovary are generally considered a variant of high grade ovarian carcinomas [44]. They are similar to serous ovarian carcinomas immunohistochemically and different from Brenner tumors [44,45]. In addition to the lacking of p63 expression as reported, we have showed in this study that transitional cell carcinomas of the ovary are consistently positive PAX8 as serous ovarian carcinomas.
Although they take on “transitional” type morphology, transitional cell carcinomas of the ovary are still composed of malignant neoplastic cells retaining the canonical Müllerian cell lineage features and might have directly derived from the fallopian tube epithelium. Our findings further indicate the difference of the cell lineage and histogenesis between Brenner tumors and transitional cell carcinomas of the ovary, which was highlighted by the mutually exclusive expression of PAX8 and p63. The significant difference may not justify classifying these two tumors in the same group.
Ovarian carcinomas with transitional cell pattern have received increased attentions recently [3,46]. Transitional cell carcinomas of ovary appeared to have a favorable response to the conventional chemotherapeutic agents and a better prognosis than other subtypes of high grade ovarian carcinomas [4-6]. Recent evidences showed that the transitional cell patterns are more frequently seen in ovarian carcinomas associated with BRCA1/2 germ line mutations. The new antitumor agents such as PARP also demonstrated promising effects on BRCA1/2 associated ovarian carcinomas [47]. Therefore, it is imperative to accurately diagnose the transitional cell carcinomas of the ovary, especially from malignant Brenner tumors. The differential diagnosis of primary malignant transitional cell tumors in the ovary has been heavily dependent on the morphological identification of benign or borderline components of Brenner tumors in the resected specimen. When the benign or borderline components are identified, the malignant transitional cell tumor is diagnosed as malignant Brenner tumors; otherwise the malignant transitional cell tumor is diagnosed as transitional cell carcinoma of the ovary. However, the benign or borderline components may not be always identified due to the overgrowth of the malignant components or inadequate sampling; on the other hand, the lower grade components of transitional cell carcinomas of the ovary may be erroneously regarded as benign or borderline components of Brenner tumors. All these may lead to the misclassification of primary malignant transitional cell tumors of the ovary. The transitional cell carcinomas are more aggressive than malignant Brenner tumors even at same stages of disease and the cell lineage differences of these two malignant tumors may convey different responses to various chemotherapeutic agents. Misclassification of these two malignant tumors might have contributed to the reported inconsistent findings on these tumors 48 and delays to define the clinical and biological features of these tumors. Therefore, we suggest a cell lineage dependent IHC approach including PAX8 and p63 to diagnose transitional cell tumors of the ovary in addition to the morphological criteria. A p63+/PAX8- transitional cell tumor in the ovary is composed of neoplastic cells of urothelial cell lineage and consistent with Brenner tumors; whereas the p63-/PAX8+ tumor is composed of neoplastic cells of Müllerian cell lineage and is consistent with transitional cell carcinomas of the ovary. These combined measures will be more accurate for the differential diagnose of malignant transitional cell tumors of the ovary, facilitate the elucidation of clinical and biological features of these tumors and selection of proper treatment regimens.
References
- Logani S, Oliva E, Amin MB, Folpe AL, Cohen C, Young RH. Immunoprofile of ovarian tumors with putative transitional cell (urothelial) differentiation using novel urothelial markers: histogenetic and diagnostic implications. Am J Surg Pathol. 2003; 27: 1434-1441.
- Austin RM, Norris HJ. Malignant Brenner tumor and transitional cell carcinoma of the ovary: a comparison. Int J Gynecol Pathol. 1987; 6: 29-39.
- Soslow RA, Han G, Park KJ, Garg K, Olvera N, Spriggs DR, et al. Morphologic patterns associated with BRCA1 and BRCA2 genotype in ovarian carcinoma. Mod Pathol. 2012; 25: 625-636.
- Robey SS, Silva EG, Gershenson DM, McLemore D, el-Naggar A, Ordonez NG. Transitional cell carcinoma in high-grade high-stage ovarian carcinoma. An indicator of favorable response to chemotherapy. Cancer. 1989; 63: 839- 847.
- Ichigo S, Takagi H, Matsunami K, Murase T, Ikeda T, Imai A. Transitional cell carcinoma of the ovary (Review). Oncol Lett. 2012; 3: 3-6.
- Gershenson DM, Silva EG, Mitchell MF, Atkinson EN, Wharton JT. Transitional cell carcinoma of the ovary: a matched control study of advanced-stage patients treated with cisplatin-based chemotherapy. Am J Obstet Gynecol. 1993; 168: 1178-1185.
- Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010; 34: 433-443.
- Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tubeperitoneal junction: a potential site of carcinogenesis. Int J Gynecol Pathol. 2011; 30: 4-11.
- Rabban JT, Crawford B, Chen LM, Powell CB, Zaloudek CJ. Transitional cell metaplasia of fallopian tube fimbriae: a potential mimic of early tubal carcinoma in risk reduction salpingo-oophorectomies from women With BRCA mutations. Am J Surg Pathol. 2009; 33: 111-119.
- Egan AJ, Russell P. Transitional (urothelial) cell metaplasia of the fallopian tube mucosa: morphological assessment of three cases. Int J Gynecol Pathol. 1996; 15: 72-76.
- Cuatrecasas M, Catasus L, Palacios J, Prat J. Transitional cell tumors of the ovary: a comparative clinicopathologic, immunohistochemical, and molecular genetic analysis of Brenner tumors and transitional cell carcinomas. Am J Surg Pathol. 2009; 33: 556-567.
- Chene G, Dauplat J, Radosevic-Robin N, Cayre A, Penault-Llorca F. Tu-be or not tu-be: that is the question… about serous ovarian carcinogenesis. Crit Rev Oncol Hematol. 2013; 88: 134-143.
- Fathalla MF. Incessant ovulation--a factor in ovarian neoplasia? Lancet. 1971; 2: 163.
- Dubeau L. The cell of origin of ovarian epithelial tumors and the ovarian surface epithelium dogma: does the emperor have no clothes? Gynecol Oncol. 1999; 72: 437-442.
- Colgan TJ. Challenges in the early diagnosis and staging of Fallopian-tube carcinomas associated with BRCA mutations. Int J Gynecol Pathol. 2003; 22: 109-120.
- Colgan TJ, Murphy J, Cole DE, Narod S, Rosen B. Occult carcinoma in prophylactic oophorectomy specimens: prevalence and association with BRCA germline mutation status. Am J Surg Pathol. 2001; 25: 1283-1289.
- Agoff SN, Garcia RL, Goff B, Swisher E. Follow-up of in situ and early-stage fallopian tube carcinoma in patients undergoing prophylactic surgery for proven or suspected BRCA-1 or BRCA-2 mutations. Am J Surg Pathol. 2004; 28: 1112-1114.
- Carlson JW, Jarboe EA, Kindelberger D, Nucci MR, Hirsch MS, Crum CP. Serous tubal intraepithelial carcinoma: diagnostic reproducibility and its implications. Int J Gynecol Pathol. 2010; 29: 310-314.
- Tone AA, Begley H, Sharma M, Murphy J, Rosen B, Brown TJ, et al. Gene expression profiles of luteal phase fallopian tube epithelium from BRCA mutation carriers resemble high-grade serous carcinoma. Clin Cancer Res. 2008; 14: 4067-4078.
- Marquez RT, Baggerly KA, Patterson AP, Liu J, Broaddus R, Frumovitz M, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005; 11: 6116-6126.
- Tong GX, Chiriboga L, Hamele-Bena D, Borczuk AC. Expression of PAX2 in papillary serous carcinoma of the ovary: immunohistochemical evidence of fallopian tube or secondary Müllerian system origin? Mod Pathol. 2007; 20: 856-863.
- Tong GX, Devaraj K, Hamele-Bena D, Yu WM, Turk A, Chen X, et al. Pax 8: a marker for carcinoma of Müllerian origin in serous effusions. Diagn Cytopathol. 2011; 39: 567-574.
- Tung CS, Mok SC, Tsang YT, Zu Z, Song H, Liu J, et al. PAX2 expression in low malignant potential ovarian tumors and low-grade ovarian serous carcinomas. Mod Pathol. 2009; 22: 1243-1250.
- Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007; 104: 331-337.
- Dubeau L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 2008; 9: 1191-1197.
- Crum CP, Drapkin R, Miron A, Ince TA, Muto M, Kindelberger DW, et al. The distal fallopian tube: a new model for pelvic serous carcinogenesis. Curr Opin Obstet Gynecol. 2007; 19: 3-9.
- Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007; 5: 35-44.
- Kurman RJ, Shih IeM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011; 42: 918- 931.
- Wang S, Qiu L, Lang JH, Shen K, Yang JX, Huang HF, et al. Clinical analysis of ovarian epithelial carcinoma with coexisting pelvic endometriosis. Am J Obstet Gynecol. 2013; 208: e1-5.
- Sayasneh A, Tsivos D, Crawford R. Endometriosis and ovarian cancer: a systematic review. ISRN Obstet Gynecol. 2011; 140310.
- Laury AR, Ning G, Quick CM, Bijron J, Parast MM, Betensky RA, et al. Fallopian tube correlates of ovarian serous borderline tumors. Am J Surg Pathol. 2011; 35: 1759-1765.
- Egan AJ, Russell P. Transitional (urothelial) cell metaplasia of the uterine cervix: morphological assessment of 31 cases. Int J Gynecol Pathol. 1997; 16: 89-98.
- Weir MM, Bell DA. Transitional cell metaplasia of the cervix: a newly described entity in cervicovaginal smears. Diagn Cytopathol. 1998; 18: 222-226.
- Lauchlan SC. Metaplasias and neoplasias of Müllerian epithelium. Histopathology. 1984; 8: 543-557.
- Mittag J, Winterhager E, Bauer K, Grummer R. Congenital hypothyroid female pax8-deficient mice are infertile despite thyroid hormone replacement therapy. Endocrinology. 2007; 148: 719-725.
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998; 2: 305-316.
- Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, Matos T, Cordon-Cardo C. Molecular pathways of urothelial development and bladder tumorigenesis. Urol Oncol. 2010; 28: 401-408.
- Ogawa K, Johansson SL, Cohen SM. Immunohistochemical analysis ofuroplakins, urothelial specific proteins, in ovarian Brenner tumors, normal tissues, and benign and neoplastic lesions of the female genital tract. Am J Pathol. 1999; 155: 1047-1050.
- Riedel I, Czernobilsky B, Lifschitz-Mercer B, Roth LM, Wu XR, Sun TT, et al. Brenner tumors but not transitional cell carcinomas of the ovary show urothelial differentiation: immunohistochemical staining of urothelial markers, including cytokeratins and uroplakins. Virchows Arch. 2001; 438: 181-191.
- Liao XY, Xue WC, Shen DH, Ngan HY, Siu MK, Cheung AN. p63 expression in ovarian tumours: a marker for Brenner tumours but not transitional cell carcinomas. Histopathology. 2007; 51: 477-483.
- Reis-Filho JS, Simpson PT, Martins A, Preto A, Gartner F, Schmitt FC. Distribution of p63, cytokeratins 5/6 and cytokeratin 14 in 51 normal and 400 neoplastic human tissue samples using TARP-4 multi-tumor tissue microarray. Virchows Arch. 2003; 443: 122-132.
- Kalebi A, Hale M. p63 expression in ovarian tumours: immunopositivity in metastatic transitional cell carcinoma of the ovary. Histopathology. 2008; 53: 228.
- Li J, Abushahin N, Pang S, Xiang L, Chambers SK, Fadare O, et al. Tubal origin of ‘ovarian’ low-grade serous carcinoma. Mod Pathol. 2011; 24: 1488- 1499.
- Ali RH, Seidman JD, Luk M, Kalloger S, Gilks CB. Transitional cell carcinoma of the ovary is related to high-grade serous carcinoma and is distinct from malignant brenner tumor. Int J Gynecol Pathol. 2012; 31: 499-506.
- Soslow RA, Rouse RV, Hendrickson MR, Silva EG, Longacre TA. Transitional cell neoplasms of the ovary and urinary bladder: a comparative immunohistochemical analysis. Int J Gynecol Pathol. 1996; 15: 257-265.
- Karnezis AN, Aysal A, Zaloudek CJ, Rabban JT. Transitional celllike morphology in ovarian endometrioid carcinoma: morphologic, immunohistochemical, and behavioral features distinguishing it from highgrade serous carcinoma. Am J Surg Pathol. 2013; 37: 24-37.
- Gan A, Green AR, Nolan CC, Martin S, Deen S. Poly(adenosine diphosphateribose) polymerase expression in BRCA-proficient ovarian high-grade serous carcinoma; association with patient survival. Hum Pathol. 2013; 44: 1638- 1647.
- Costa MJ, Hansen C, Dickerman A, Scudder SA. Clinicopathologic significance of transitional cell carcinoma pattern in nonlocalized ovarian epithelial tumors (stages 2-4). Am J Clin Pathol. 1998; 109: 173-180.
