Abstract
Switchgrass is one of the most promising bioenergy crops for the US. It produces relatively high biomass yield and can grow on marginal lands. However, some traits need to be improved, including stand establishment and stress tolerance. We previously reported that Burkholderia phytofirmans PsJN promoted growth of switchgrass cv. Alamo. However, PsJN was found to be genotype specific in promoting switchgrass growth. In an effort to identify endophytes which have a broad spectrum of growth promotion with more efficiency, we isolated a bacterium from surface-sterilized switchgrass seeds. The bacterium was identified as a Pantoea agglomerans species and named strain PaKM. Our experiments demonstrated that PaKM significantly boosts switchgrass growth/vigor and root system development, and increases biomass yield under in vitro, growth chamber, greenhouse, as well as field conditions. Not only does PaKM promote growth of switchgrass Alamo, but also 8 other switchgrass cultivars tested. PaKM also enhanced salt and drought stress tolerance in vitro. Assays demonstrated that PaKM has the ability to solubilize phosphate and produces high levels of auxin. In summary, PaKM is an efficient growth promoter of switchgrass over a broad spectrum of genotypes and has potential application with low input and sustainable production systems on marginal lands.
Keywords: Bacterial endophyte; Broad spectrum of growth promotion; Auxin level; Mechanisms; Pantoea agglomerans strain PaKM; Switchgrass
Introduction
While the economies of developing and developed countries continue to grow and improve, petroleum oil demand will only increase. As consumption of fossil fuels causes environmental pollution and global warming, it is imperative to find clean alternative energy sources, especially renewable energy for the future. Bioenergy is one such source as it is synthesized by higher plants and alga by utilizing sunlight and carbon dioxide from the atmosphere. The use of bioenergy not only has potential to greatly reduce greenhouse gas emissions in an environmentally friendly way, but it is expected to also boost rural economies. Switchgrass is considered one of the most promising bioenergy crop candidates in the US because it is a perennial warm-season grass native to North America, can grow in poor soil, and requires little fertilizer and pesticide inputs. However, consistent improvements in biomass yield and sustainability are needed, and poor stand establishment in the first year is an issue. Much effort is directed towards overcoming these challenges to develop a low input and sustainable perennial feedstock production system capable of producing reliably high biomass yields on marginal lands.
There are many approaches to improve switchgrass biomass yields including conventional breeding and biotechnology. However, these approaches may take a decade or longer to develop new cultivars. The use of beneficial endophytes, which reside in plant tissues and cause no apparent harm, is a practical and feasible way to increase switchgrass biomass yield and enhance stress tolerance [1,2]. Also, beneficial endophytes are naturally occurring, and their widespread use will likely face few restrictions. Endophytes generally promote host plant growth, enhance nutrient uptake, increase stress tolerance, and inhibit plant pathogen growth [1,3,4]. These benefits are wide ranging across a broad spectrum of plants. For example, one of the most studied plant growth promoting endophytes, Burkholderia phytofirmans strain PsJN, stimulates growth of many plant species, including potato, tomato, grapevine, and switchgrass [5-10]. Many other examples have been reported for endophyte-mediated plant growth promotion. Endophytic Klebsiella oxytoca strain GR-3 promoted the growth of Typha australis, a semi-aquatic grass [11]. Two isolates, PS4 and PS27, closely related to Pseudomonas rhodesiae and Pantoea ananatis respectively, successfully colonized pepper roots and significantly promoted plant growth and enhanced root fresh weight by 73.9% and 41.5% respectively [12].
Research on bacterial endophytes in switchgrass has increased in recent years. For example, Gagne-Bourgue et al. [13] isolated and characterized 31 bacterial endophytes from leaves of three switchgrass cultivars. Xia et al. [14] characterized a total of 307 bacterial isolates from surface sterilized switchgrass shoots, roots and seeds, and tested their abilities to influence plant growth. Ker et al. [4] isolated 8 bacterial strains from Cave-in-Rock rhizomes and inoculated switchgrass seeds with a mixture of 8 strains in a low N field and observed 40% yield increase in the first establishment year. We have tested Burkholderia phytofirmans strain PsJN in switchgrass and found that PsJN significantly promoted switchgrass cv. Alamo growth under in vitro, growth chamber, and greenhouse conditions [9], as well as in the field [10]. However, the growth promotion was genotype specific, as the upland switchgrass cv. Cave-in-Rock exhibits no growth promotion by PsJN [9]. There have been several reports regarding genotypic specificity of endophytes [15,16]. Therefore, exploring beneficial endophytes having a broad spectrum of growth promotion is imperative and has potential in practical applications in sustainable bioenergy feedstock production. In this study, we report the isolation and identification of a bacterial endophyte, PaKM, from surface-sterilized switchgrass seeds, and demonstrated its growth promotion under in vitro, greenhouse and field conditions. We also characterized its possible mechanisms of growth promotion.
Materials and Methods
PaKM isolation and identification
A yellow bacterium was found in surface-sterilized switchgrass seeds growing on MS medium (M519, PhytoTechnology Laboratories, Overland Park, KS, USA). After its ability to enhance switchgrass growth was confirmed, a single colony was isolated and sent to Midi Labs in Newark, DE for species identification with 16S rDNA gene sequence similarity and FAME matches.
Switchgrass seed sterilization
Switchgrass (Panicum virgatum L.) cvs. Alamo and Cave-in-Rock seeds were purchased from Warner Brothers Seed Co. (Lawton, OK, USA). Other cultivars were kindly provided by Dr. Bingyu Zhao (Department of Horticulture, Virginia Tech). Switchgrass seed surface-sterilization followed our previous report [9].
Bacterial endophyte culture conditions
The cultures were streaked on Luria Broth (LB) solid medium. Inoculum was produced by transferring one loop of PaKM from 1-day-old cultures to 5 ml LB broth in a 15-ml culture tube, followed by incubation at 28°C on a shaker (200 rpm) overnight. Five ml of the overnight PaKM cultures were added to 45 ml LB medium in a 250- ml Erlenmeyer flask and grown to 0.7 at OD600. Bacterial cells were then collected by centrifugation at 3,500 rpm for 7 min at 4°C, and re-suspended in PBS buffer (10 mM NaH2PO4, 0.8% NaC1, pH 6.5) after which the OD600 was adjusted with PBS buffer to 0.5.
PaKM tagged with GFP for visualization of colonization
In order to visualize colonization of PaKM in plants, PaKM wild type was transformed with plasmid p519ngfp (ATCC® Cat. No. 87453, Manassas, Virginia, USA) using electroporation. The transformants were selected on solid LB medium supplemented with 50 mg/l kanamycin and cultured at 37°C for 2 days. The colonies with GFP were chosen under a fluorescent microscope.
PaKM colonization
The plants inoculated with PaKM-GFP were examined under a fluorescent stereomicroscope equipped with a GFP filter (BP460- 490) (Model SZXILLD2-100; Olympus, Tokyo, Japan) to observe colonization. For bioassays, the control and PaKM-GFP inoculated plants were surface-sterilized with 0.032 M sodium hypochlorite for 1 min, then washed 4X with sterile deionized, distilled water (ddH2O). Fifty μl of the final wash were plated on solid LB medium to confirm effectiveness of surface sterilization. Root, leaf and sheath parts were then separated, weighed, and ground with mortar and pestle in 1 ml sterile ddH2O. The homogenates were then centrifuged at 2000 rpm for 3 min, and the supernatant diluted to 1:10, 1:100, and 1:1000 with sterile ddH2O. Fifty μl samples of the serially diluted solutions were spread on solid LB medium. The plates were incubated for 2 days at 28oC in the dark, and the number of GFP-positive colonies determined under fluorescence stereomicroscopy.
PaKM inoculation and plant growth conditions
Surface-sterilized seeds were germinated on wet filter paper in petri-dishes for 4-6 days at 25°C under white fluorescent light (67 μmol m-2 s-1), 16 h photoperiod. The root tips of the young seedlings were cut prior to PaKM inoculation to facilitate bacterial penetration, and seedlings were inoculated by soaking in PaKM solution (0.5 of OD600) for 1 min. Control seedlings were treated with PBS buffer alone. The treated seedlings were blot-dried with sterile paper towel, placed in GA7 Magenta vessels (Sigma-Aldrich) containing 50 ml of MS medium, 30 g/l maltose and 3 g/l phytogel, pH 5.8. In each vessel, 5 seedlings were placed and grown for 25-30 days in the incubator as above. Root and shoot length, and seedling fresh weight were then determined, and the plants transferred to a soil mix composed of 2/3 Miracle-Gro Potting Mix (Scotts Miracle-Gro Company, Marysville, Ohio, USA) and 1/3 Arabidopsis growing media (Lehle Seeds, Round Rock, Texas, USA). Plants were grown in 72-cavity trays in a growth chamber at a 28/22°C day/night temperature, 16 h photoperiod (white fluorescent bulbs at 88 μmol m-2 s-1) for 30 days, or 4-gallon pots in the greenhouse.
Salt and drought tolerance
Salt and drought tolerances were tested in vitro using PaKM inoculated and non-inoculated Alamo seedlings. Seed sterilization, germination and PaKM inoculation were described above. Inoculated seedlings and controls were then placed in salt stress medium (MS + 100 mM NaCl) or drought stress medium (MS + 7.52 g/L of D-mannitol, which leads to -0.4 MPa), and grown in a growth chamber under the above conditions for 40 days.
Field trial experimental design
The low fertility soil site was chosen at Walden Farm, a historic tobacco farm near Danville, Virginia, USA. Levels of P, K, Ca, and Mg were rated Low, Medium -, Low +, and High, respectively [10]. Additionally, no fertilizers were applied before or during the trial, and only one initial watering was performed at the time of transplanting. A paired experimental design was carried out to reduce soil and environmental variation. The distance between plants and the space between rows were both at 0.76 meter. Seedlings were inoculated following above procedure on July 3, 2012 and transplanted in the field on August 20, 2012. During the second year, root and shoot growth was determined on June 17, 2013 (n=10) after digging up the entire plants and washing roots with tap water. Roots were then blot dried with paper towels, and fresh weights of roots and shoots determined. The plants were then dried at ambient indoor environment (21°C and 35% humidity) for two weeks, and dry weights recorded. Fourteen pairs of aboveground parts were harvested on December 04, 2013 after the plants were dormant.
Root morphology
To determine the effect of PaKM inoculation on root growth and morphology, bacterized and non-bacterized Alamo plants were transplanted in 4-gallon pots containing Miracle Gro® potting mix on March 28, 2013 in a temperature controlled greenhouse at the Institute for Advanced Learning and Research in Danville, Virginia and the entire plant harvested on May 14, 2013. Roots were washed, and fresh and dry weights of roots and shoots determined. The numbers of lateral roots per cm on seminal roots were estimated by counting lateral roots in a 3 cm portion of a randomly selected seminal root and dividing by 3 [10]. The number of seminal roots was also counted on PaKM and control plants as well.
Growth promotion of different switchgrass cultivars by PaKM
Seven different switchgrass cultivars (Forestburg, Nebraska, Shawnee, Blackwell, Shelton, Sunburst and Canthage) were tested for plant growth promotion by PaKM in vitro. Seed surface sterilization and growth conditions were described previously [9].
Auxin quantification
The auxin quantification method was modified from Patten and Glick’s protocol [17]. PaKM bacteria were cultured in 4 ml of LB medium at 200 rpm, 30°C overnight. Twenty μl of overnight bacterial cultures were aliquoted into 4 ml of LB medium containing 100 μg/ ml of L- tryptophan. The cultures were incubated at 30oC for 2 days and centrifuged at 14,000 rpm for 10 min. The supernatant (0.5 ml) was mixed well with 2 ml of Salkowski reagent (15 ml of concentrated H2SO4, 25 ml of ddH2O and 0.75 ml of 0.5 M FeCl3.6H2O) and kept at room temperature for 20 min before measuring absorbance at 540 nm using Beckman Coulter Multimode DTX 880 (Beckman Coulter, Inc., Fullerton, CA, USA). Indole Acetic Acid (IAA) standard curve was prepared using 0.5 ml of IAA at different concentrations (0, 20, 40, 60, 80, 100 μg/ml) following the same procedure described above. The auxin concentration was expressed as μg IAA/mg fresh weigh of bacterial cells.
ACC (1-Aminocyclopropane-1-Carboxylate) deaminase activity
The ACC deaminase activity was measured following Penrose and Glick’s protocol [18]. The absorbance was measured at 540 nm with a Beckman Coulter Multimode DTX 880.
Phosphate solubilization and soluble P quantification
One μl of overnight growing PaKM was placed in the center of Pikovskaya’s agar medium plate, incubated at 30°C and a clear zone (halo) was measured at 6 days. Soluble P was also quantified with Murphy and Riley [19] method. Briefly, one hundred μl of overnight PaKM culture was added to 3.9 ml of NBRIP liquid medium [20] and grown at 30°C, 200 rpm for 3 days. Then liquid culture was centrifuged at 14,000 rpm for 10 min, and soluble P in the supernatant was quantified at 840 nm. The soluble P standard curve was established with KH2PO4.
Statistical analyses
Statistical analyses were performed using student’s t-test except for field experiment. For the field trial, a student’s paired t-test was carried out to reduce soil and environmental variation. Values were assigned to each group and reported at 95%, 99%, or 99.9% confidence levels.
Results
PaKM isolation and identification
The bacterium isolated from surface sterilized seeds was characterized as a yellow gram negative rod shaped bacterium. Based on data from 16S rDNA gene sequence similarity and FAME matches done by Midi Labs in Newark, DE, it was identified to belong to species Pantoea agglomerans (Table 1) and labeled as strain PaKM.
Fame Matches
Similarity Index
Entry Name
0.794
Pantoea-agglomerans-GC subgroup B
0.638
Raoultella-terrigena (Klebsiella)
0.611
Kluyvera-cryocrescens-GC subgroup B
16S DNA Match
% Diff
Length
Library Entry Name
0.28
528
Pantoea-agglomerans
1.52
526
Pantoea-ananatis
3.69
528
Citrobacter-freundii
Cross Library Report
% Diff
Genus
Species
Fame SI
0.28
Pantoea
agglomerans
0.794
1.52
Pantoea
ananatis
0.221
3.98
Citrobacter
freundii
0.499
Table 1: PaKM identification with 16S rDNA sequencing and FAME matches.
PaKM colonization
To visualize PaKM infection and colonization under a fluorescent microscope, we transformed it with the plasmid p519ngfp (ATCC #87453). Figure 1A revealed PaKM-GFP colonization of the interior of switchgrass roots 7 days after inoculation. A series of biological assays indicated PaKM infected and colonized roots first, followed by translocation to the sheath and leaves (Figure 1B).
Growth promotion in vitro
When seedlings derived from sterilized and imbibed seeds were inoculated with PaKM and grown in vitro, the PaKM-inoculated plants were significantly larger compared to the non-inoculated controls. The PaKM inoculated seedlings were more vigorous, and their stem base became dark brown. The leaves were also greener compared to the controls (Figures 1C & 1D). Furthermore, PaKM significantly enhanced growth of the upland cv. Cave-in-Rock, with a 197% increase in total fresh weight compared with the control plants (p<0.001). This cultivar did not respond to inoculation with a commonly studied plant growth promoting bacterial endophyte, Burkholderia phytofirmans strain PsJN [9].
Growth promotion in growth chamber
After PaKM-inoculated plants were grown in vitro for one month, the plants were transferred to a flat with 72 cavities filled with soil and grown in growth chamber for another month. The results showed that the PaKM inoculation maintained growth advances in both Alamo and Cave-in-Rock, and the total fresh and dry weights were significantly higher in PaKM-inoculated plants compared to the control plants (Figure 2).
When comparing the degree of fresh weight increase with that of dry weight increase, we found dry weight increases were greater than fresh weight increases. For example, the total fresh weight of Alamo and Cave-in-Rock was increased by 44% and 117%, respectively while the total dry weight increased by 61% and 143%, respectively. This data implies that PaKM could improve photosynthesis and increase switchgrass biomass.
Growth promotion in the greenhouse and field with low fertility soil
Similar growth promotion effect caused by PaKM-inoculation was recorded in the greenhouse experiment with 4-gallon pots (Figure 3A). Two months after transplanting total dry weights of Alamo and Cave-in-Rock were significantly higher than controls (108% and 105% increase, respectively).
The PaKM stimulation of plant growth was also confirmed in a field experiment on a low nutrient soil. Compared to the noninoculated controls, the PaKM bacterized plants had significantly higher shoot and root biomass (p=0.03 and 0.04, respectively) at midseason of the second year growth (56% increase for shoots and 75% increase for roots over controls). The PaKM growth enhancing effect persisted until the end of the second season. Aboveground biomass of the bacterized plants was 31% higher compared to control (Figure 3B).
Root growth, morphology and tiller number
PaKM-inoculated plants produced more primary roots (83%) and lateral roots (73%) than controls in the greenhouse pot study (Figure 4). The inoculated plants had also longer roots (110% longer than controls). PaKM-inoculated plants produced more tillers, especially early tillers, compared to the non-inoculated controls. PaKMinoculated Alamo had 170% and 20% more tillers at one month and two months after transplanting compared with controls, respectively (Figure 4).
Salt and drought tolerance in vitro
PaKM-inoculated Alamo seedlings and controls were grown in salt or drought stress media under in vitro conditions for 40 days. The results showed that PaKM-inoculated plants had a 3-fold and 2.7- fold increase in fresh weight over controls in salt and drought stress media, respectively (Figure 5).
Comparison of PaKM with PsJN in growth promotion
Previously, we tested one of most studied plant growth promoting bacteria, Burkholderia phytofirmans strain PsJN and found that it significantly promoted switchgrass cv. Alamo growth under in vitro, growth chamber, and greenhouse conditions [9]. We compared growth promotion by PaKM with PsJN in switchgrass cv. Alamo under both in vitro and growth chamber conditions. The total fresh weight was 2 times higher in PsJN and 8 times higher in PaKM inoculated Alamo compared to non-inoculated control plants in vitro (Figure 6A). When the above plants were transplanted to 72-cavity trays and grown in a growth chamber for another month, the total fresh weights of PsJN- and PaKM-inoculated plants were 2.2 times and 4.6 times over non-inoculated control plants, respectively (Figure 6B). The total dry weights of PsJN- and PaKM-inoculated plants were 2.0 times and 4.9 times over the control plants, respectively (Figure 6B).
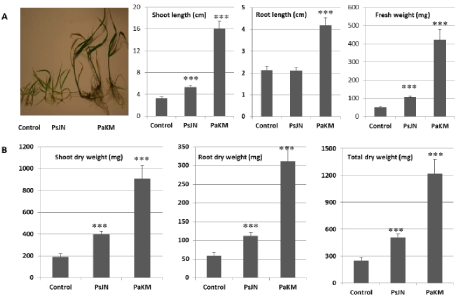
Figure 6: Comparison of PaKM with PsJN in growth promotion of Alamo in vitro and in greenhouse. A) Picture and statistic data for plants grown in vitro for 54
days. B) Plant dry weight after plants grew in vitro for 54 days and transferred to soil and grew in greenhouse.
Response of different switchgrass cultivars to PaKM
Our previous experiments indicated that PsJN growth promotion is genotypic specific as PsJN does not promote growth in switchgrass upland cv. Cave-in-Rock [9]. Our results demonstrated that PaKM not only promotes growth in switchgrass lowland cv. Alamo but also upland cv. Cave-in-Rock (Figure 3). In order to determine if PaKM demonstrates a broad spectrum of growth promotion across switchgrass genotypes, we tested an additional 7 cultivars (Forestburg, Nebraska, Shawnee, Blackwell, Shelton, Sunburst, and Canthage) under in vitro conditions. (Table 2) exhibits that PaKM significantly promoted growth of all cultivars, and the total fresh weight increased from 77% to 238% in PaKM-inoculated plants over their corresponding control plants.
Cultivars
Treatment
Root (cm)
Shoot (cm)
Total (mg)
% of Control
Shawnee
Control
PaKM
p-value
2.5
3.8
2.30E-03
7
15.1
1.80E-06
31.6
84.7
4.50E-05
268
Nebraska
Control
PaKM
p-value
1.9
2.3
1.20E-01
10.1
16.5
9.40E-06
34.8
86.1
3.60E-05
247
Forestburg
Control
PaKM
p-value
2
1.3
2.40E-02
8.8
11.4
1.00E-02
32.6
57.7
4.30E-03
177
Shelton
Control
PaKM
p-value
3
3.2
3.50E-01
12.2
11.4
3.10E-01
43.6
141.2
1.20E-02
324
Blackwell
Control
PaKM
p-value
2.4
3.9
1.90E-02
10.6
15.4
1.00E-02
45.9
122.4
4.60E-04
267
Sunburst
Control
PaKM
p-value
1.6
4.1
3.40E-05
9.8
15
2.60E-03
37
125.4
2.70E-04
338
Canthage
Control
PaKM
p-value
2.1
3.3
4.60E-03
8.5
18
6.60E-06
29.7
86
2.60E-04
290
Cave-in-Rock
Control
PaKM
p-value
2.3
3.6
7.50E-07
2.7
8.3
7.80E-19
25.3
75.2
2.50E-11
297
Table 2: PaKM significantly promoted growth of various switchgrass cultivars.
Auxin level and ACC deaminase activity of PaKM
Auxin is a plant growth promoting hormone and plays important roles in growth and development. The ability of PaKM to produce auxin was determined. The results demonstrated that PaKM produces a higher level of auxin (5.92±0.19 μg/mg fresh weight of cells) compared to PsJN (0.58±0.03). However, PaKM has no ACC deaminase activity, indicating that the two bacteria promote switchgrass growth by different mechanisms.
Phosphate solubilization
When PaKM grew in Pikovskaya’s agar medium for 6 days, the clear zone (halo) was observed with a diameter at 1.8 cm and colony size at 0.8 cm. When PaKM was cultured in NBRIP liquid medium for 3 days, the soluble P concentration was 287 μg/ml.
Discussion
As concerns about greenhouse gas emissions from fossil fuel use and global warming increase, options for clean and sustainable energy sources need to be explored. Bioenergy from biomass is a primary area of focus. Switchgrass is a promising feedstock and has been the focus of multiple studies regarding biomass yield improvement [21-23]. Utilizing beneficial microorganisms which form intimate associations with plants and promote growth and health, is one such approach to increase yield [24,25]. In this study, we explored growth promotion under a variety of conditions, colonization, and the underlying mechanisms of action of PaKM, an endophyte isolated from switchgrass seed. To verify its endophytic lifestyle, we visualized colonization under a fluorescent microscope with GFP tagged PaKM, and quantified PaKM inside plants with biological assays (Figure 1). These investigations demonstrated that PaKM infected and colonized switchgrass through the root and moved vertically and sequentially to the sheath and leaves. Similar results were reported when the well-studied bacterial endophyte PsJN was inoculated into switchgrass [9], grapevine [26], and potato [27]. In each case, PsJN was transported through the interior vascular system from root xylem vessels to the upper parts of the plants, a critical first step in the endophytic bacterium-plant interaction [28]. PaKM exhibited a similar colonization pattern.
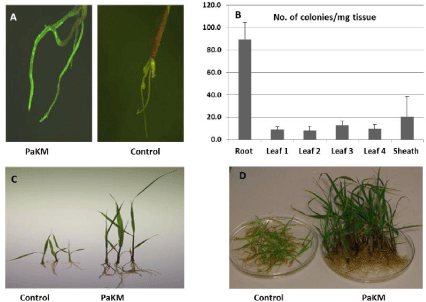
Figure 1: PaKM colonization and growth promotion in switchgrass cv. Alamo. A) PaKM-GFP was visualized after 7-day inoculation under fluorescence microscope.
B) Biologic assay for PaKM colonization in roots, sheath and leaves. C) Picture for 3 representative seedlings, and D) Picture for population group grown in vitro
for one month after inoculated with PaKM.
We confirmed that PaKM promotes growth of switchgrass under in vitro, growth chamber, greenhouse, and field conditions (Figures 2 & 3) due in part to an increased number of early tillers and the establishment of large root systems (Figure 4). Increased tillering is an important early indicator of stand establishment and future biomass production potential in perennial grasses [29,30], and larger root systems allow more access to water and nutrients and also help plants better tolerate drought conditions [31] and enhance their ability to manage water [4]. Both findings are important to early growth vigor and switchgrass seedling year establishment.
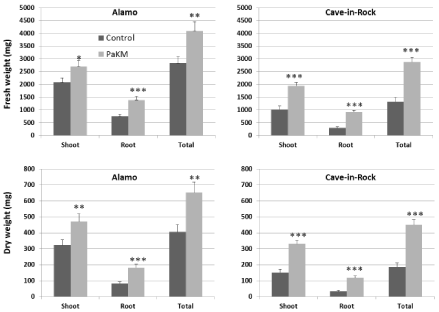
Figure 2: PaKM greatly promoted growth of switchgrass cvs. Alamo and Cave-in-Rock in a growth chamber. Seedlings were inoculated with PaKM and grown in
vitro for 3 weeks and then transplanted in flats with soil and grown in a growth chamber for one month. Significant differences between control and PaKM inoculated
plants were determined using t-test (*p< 0.05, **p<0.01, and ***p<0.001).
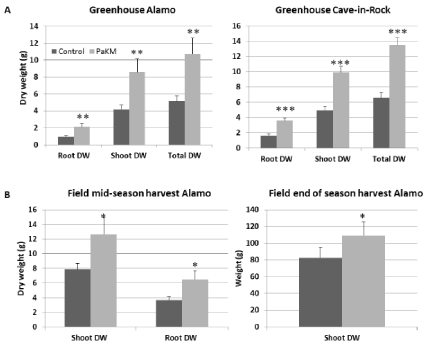
Figure 3: PaKM greatly promoted growth of switchgrass cvs. Alamo and Cave-in-Rock in the greenhouse (A) and the field (B). Significant differences between
control and PaKM inoculated plants were determined using t-test (*p< 0.05, **p<0.01, and ***p<0.001).
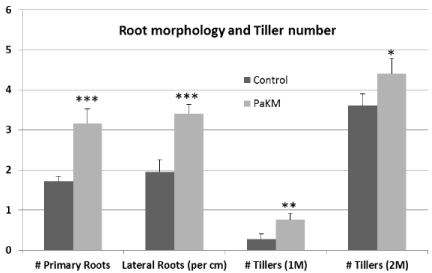
Figure 4: PaKM significantly increased the numbers of primary and lateral roots and the number of tillers. Significant differences between control and PaKM
inoculated plants were determined using t-test (*p< 0.05, **p<0.01, and ***p<0.001).
A variety of mechanisms have been reported under which beneficial bacterial endophytes promote plant growth, such as nitrogen fixation, phosphate solubilization, siderophore secretion, and phytohormone production [32]. In this study, we found that PaKM has the ability to produce high levels of auxin, which can stimulate cell elongation and cell division, and causes growth promotion in host plants. Many bacterial endophytes have been reported to have the ability to produce IAA [33,34]. PaKM was also shown to solubilize phosphate, another potential mechanism for growth promotion. In contrast, Burkholderia phytofirmans strain PsJN promotes host plant growth by producing high level of ACC deaminase activity, in turn reducing ethylene levels to promote plant growth [35].
In switchgrass, we reported that PsJN significantly promoted switchgrass growth. However, PsJN growth promotion is genotype specific, and cv. Cave-in-Rock does not show any growth promoting effect [9]. Similar genotype responsiveness has been reported in other species [15,16]. For example, it was reported that the potato response to PsJN involves some form of genetic control, since some potato cultivars display the beneficial response to the endophyte, while others do not [36-38]. In the present study, we found that PaKM significantly promoted growth of Alamo and Cave-in-Rock, as well as 7 additional switchgrass cultivars (Table 2). Therefore, PaKM has a broad spectrum of growth promotion in switchgrass, with possible enhancement of stress tolerance (Figure 5). Utilizing bacterial endophytes broadly and practically is inhibited by genotype specificity. The broad spectrum of switchgrass genotypic growth promotion by endophytes like PaKM will increase the potential for the development of low input and sustainable switchgrass feedstock production systems.
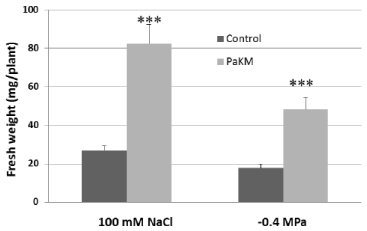
Figure 5: PaKM significantly enhanced salt and drought tolerance of switchgrass cv. Alamo in vitro. Seedlings were inoculated with PaKM and then grown in salt
or drought stress media for 40 days. Significant differences between control and PaKM inoculated plants were determined using t-test (***p<0.001).
Conclusion
Pantoea agglomerans strain PaKM, a naturally-occurring bacterium, was isolated from surface-sterilized switchgrass seeds. It proved to be an efficient growth promoter of switchgrass over a broad spectrum of genotypes and enhanced salt and drought stress tolerance in vitro. PaKM significantly boosted root system development, and increased biomass yield under in vitro, growth chamber, greenhouse, as well as field conditions. A primary mechanism for growth promotion by PaKM is to produce high levels of auxin, a plant growth promotive hormone. In summary, PaKM has potential use as a biostimulant to promote growth of the renewable bioenergy crop switchgrass, particularly on marginal lands.
Acknowledgements
The authors thank Dr. Jerzy Nowak for instrumental suggestions during this research and critical writing editing. This work was funded through Special Grants (2003-38891-02112, 2008-38891- 19353 and 2009-38891-20092) and HATCH funds (Project No. VA-135816) from the United States Department of Agriculture, the Office of Science (BER), U.S. Department of Energy for Plant Feedstock Genomics for Bioenergy Program (DE-SC0004951), and operating funds from the Commonwealth of Virginia to the Institute for Advanced Learning and Research.
References
- Mei C, Lava-Chavez A, Lowman SJ, Flinn BS. The use of endophytes and mycorrhizae in switchgrass for biomass production. Luo H, Wu Y, Kole C, Editors In: Compendium of Bioenergy Plants: Switchgrass. The Science publishers, Inc. (New Hampshire) jointly with CRC Press of Taylor and Francis Group. 2014; 67-108.
- Farrar K, Bryant D, Cope-Selby N. Understanding and engineering beneficial plant-microbe interactions: plant growth promotion in energy crops. Plant Biotechnol J. 2014; 12: 1193-1206.
- Mei C, Flinn BS. The use of beneficial microbial endophytes for plant biomass and stress tolerance improvement. Recent Pat Biotechnol. 2010; 4: 81-95.
- Ker K, Seguin P, Driscoll BT, Fyles JW, Smith DL. Switchgrass establishment and seeding year production can be improved by inoculation with rhizosphere endophytes. Biomass Bioenergy. 2012; 47: 295-301.
- Lazarovits G, Nowak J. Rhizobacteria for improvement of plant growth and establishment. Hort Science. 1997; 32: 188-192.
- Sharma VK, Nowak J. Enhancement of verticillium wilt resistance in tomato transplants by in vitro co-culture of seedlings with a plant growth promoting rhizobacterium (Pseudomonas sp. strain PsJN). Can J Microbiol. 1998; 44: 528-536.
- Nowak J, Bensalim S, Smith CD, Dunbar C, Asiedu SK, Madani A, et al. Behavior of plant material issued from in vitro tuberization. Potato Res. 1999; 42: 505-519.
- Ait Barka E, Nowak J, Clement C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl Environ Microbiol. 2006; 72: 7246-7252.
- Kim S, Lowman S, Hou G, Nowak J, Flinn B, Mei C. Growth promotion and colonization of switchgrass (Panicum virgatum) cv. Alamo by bacterial endophyte Burkholderia phytofirmans strain PsJN. Biotechnol Biofuels. 2012; 5: 37.
- Lowman JS, Lava-Chavez A, Kim-Dura S, Flinn B, Nowak J, Mei C. Switchgrass field performance on two soils as affected by bacterization of seedlings with Burkholderia phytofirmans strain PsJN. BioEnergy Res. 2015; 8: 440-449.
- Jha PN, Kumar A. Endophytic colonization of Typha australis by a plant growth-promoting bacterium Klebsiella oxytoca strain GR-3. J Appl Microbiol. 2007; 103: 1311-1320.
- Kang SH, Cho HS, Cheong H, Ryu CM, Kim JF, Park SH. Two bacterial entophytes eliciting both plant growth promotion and plant defence on pepper (Capsicum annuum L.). J Microbiol Biotechnol. 2007; 17: 96-103.
- Gagne-Bourgue F, Aliferis KA, Seguin P, Rani M, Samson R, Jabaji S. Isolation and characterization of indigenous endophytic bacteria associated with leaves of switchgrass (Panicum virgatum L.) cultivars. J Appl Microbiol. 2013; 114: 836-853.
- Xia Y, Greissworth E, Mucci C, Williams MA, De Bolt S. Characterization of culturable bacterial endophytes of switchgrass (Panicum virgatum L.) and their capacity to influence plant growth. GCB Bioenergy. 2013; 5: 674-682.
- Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, Weyens N, et al. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol. 2009; 75: 748-757.
- Da K, Nowak J, Flinn B. Potato cytosine methylation and gene expression changes induced by a beneficial bacterial endophyte, Burkholderia phytofirmans strain PsJN. Plant Physiol Biochem. 2012; 50: 24-34.
- Patten CL, Glick BR. Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol. 2002; 68: 3795-3801.
- Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003; 118: 10-15.
- Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962; 27: 31-36.
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999; 170: 265-270.
- Shen H, He X, Poovaiah CR, Wuddineh WA, Ma J, Mann DGJ, et al. Functional characterization of the switchgrass (Panicum virgatum) R2R3- MYB transcription factor PvMYB4 for improvement of lignocellulosic feedstocks. New Phytol. 2012; 193: 121-136.
- Mitchell RB, Vogel KP, Sarath G. Managing and enhancing switchgrass as a bioenergy feedstock. Biofuel Bioprod Biorefin. 2008; 2: 530-539.
- McLaughlin SB, de la Torre Ugarte DG, Garten CT Jr, Lynd LR, Sanderson MA, Tolbert VR, et al. High-value renewable energy from prairie grasses. Environ Sci Technol. 2002; 36: 2122-2129.
- Berg G. Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbial Biotechnol. 2009; 84: 11-18.
- Sturz AV, Christie B, Nowak J. Bacterial endophytes: potential roles in developing sustainable systems of crop production. Crit Rev Plant Sci. 2000; 19: 1-30.
- Compant S, Reciter B, Sessitsch A, Nowak J, Clement C, Barka EA. Endophytic colonization of Vitis vinifere L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microbiol. 2005; 71: 1685-1693.
- Reiter B, Pfeifer U, Schwab H, Sessitsch A. Response of endophytic bacterial communities in potato plants to infection with Erwinia caratovora subsp. atroseptican. Appl Environ Microbiol. 2002; 68: 2261-2268.
- Whipps JM. Microbial interactions and biocontrol in the rhizosphere. J Exp Bot. 2001; 52: 487-511.
- Boe A, Beck DL. Yield components of biomass in switchgrass. Crop Sci. 2008; 48: 1306.
- Dobbelaere S, Croonenborghs A, Thys A, Ptacek D, Vanderleyden J, Dutto P. Responses of agronomically important crops to inoculation with Azospirillum. Aust J Plant Physiol. 2001; 28: 871-879.
- Mehnaz S, Lazarovits G. Inoculation effects of Pseudomonas putida, Gluconacetobacter azotocaptans, and Azospirillum lipoferum on corn plant growth under greenhouse conditions. Microb Ecol. 2006; 51: 326-335.
- Ahemad M, Kibret M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J King Saud U - Sci. 2014; 20: 1-20.
- Hayat R, Ali S, Amara U, Khalid R, Ahmed I. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010; 60: 579-598.
- Montanez A, Rodriguez-Blanco A, Barlocco C, Beracochea M, Sicardi M. Characterization of cultivable putative endophytic plant growth promoting bacteria associated with maize cultivars (Zea mays L.) and their inoculation effects in vitro. Appl Soil Ecol. 2012; 58: 21-28.
- Sun Y, Cheng Z, Glick BR. The role of 1-Aminocyclopropane-1-Carboxylate (ACC) deaminase in plant growth promotion by the endophytic bacterium Burkholderia phytofirmans PsJN. FEMS Microbiol Lett. 2009; 296: 131-136.
- Bensalim S, Nowak J, Asiedu SK. A plant growth promoting rhizobacterium and temperature effects on performance of 18 clones of potato. Am J Potato Res. 1998; 75: 145-152.
- Nowak J, Asiedu SK, Bensalim S, Richards J, Stewart A, Smith C, et al. From laboratory to applications: challenges and progress with in vitro dual cultures of potato and beneficial bacteria. Plant Cell Tiss Org Cult. 1998; 52: 97-103.
- Nowak J, Veilleux RE, Nowak J, Turgeon S. Priming for transplant stress resistance in in vitro propagation via plantlet bacterization. Acta Hort. 2007; 748: 65-75.
