Abstract
Background: Colletotrichum gloeosporioides is important plant pathogens on a wide range of plant hosts such as citrus causing pre- or post-harvest infections as anthracnose, post-bloom fruit drop, tearstain and stem-end rot on fruit, or wither-tip of twigs.
Method: The optimization of growth conditions of this pathogen was performed (solid media, temperature, pH and water potential under laboratory experiments).
Results: Our results revealed that the maximum radial growth of C. gloeosporioides was recorded on SDA medium. All isolates were able to grow on PDA at temperatures of 15 and 30°C (over 0.7cm/day). Optimal growth radial was recorded at pH 5, 6, 7 and 8. Similar responses were obtained with both salt types, but, in general, C. gloeosporioides was more tolerant to KCl than NaCl.
Conclusion: Studies of cultural, morphological traits of the pathogen are prominent to understand the response of the pathogen in different environmental and nutritional conditions.
Keywords: Anthracnose; Citrus; Colletotrichum gloeosporioides; Osmotic potential
Background
In Tunisia, Cap-bon area is the main location for citrus with more than 70% of the national production. The local market absorbs 80 to 90% of the production [1]. The most cultivated varieties are the oranges Thomson, Meski and Valencia late (Citrus sinensis), the clementines (Citrus reticulata) and the lemons (Citrus limon) [2]. Therefore, the study and knowledge of all the pathogens affecting this crop is imperative. The use of a polyphasic approach in the past revealed new Colletotrichum species associated with citrus [3]. Colletotrichum gloeosporioides was previously thought to be the only Colletotrichum species causing post-harvest anthracnose [4-6], but investigations that are more recent showed that several species of Colletotrichum are associated with fruit decay worldwide [7-10]. Recently, various infections caused by Colletotrichum spp. strongly compromised citrus production in different Mediterranean countries. In fact, heavy pre-harvest anthracnose symptoms appeared on orange fruits and lesions on leaves of mandarins in Italy [11,12], twig withertip symptoms were observed on cultivated orange trees in Tunisia [13], and severe anthracnose symptoms on unripe and ripe lemon fruits were recorded in Portugal [14]. The optimal development conditions of C. gloeosporioides require 25-28°C temperature, pH 5.8-6.5. This pathogen is inactive in dry season and switches to active stages when encountered favorable environmental conditions [15]. Various medium preparations were employed for the growth and sporulation of C. gloeosporioides including Potato dextrose agar, lima bean agar, malt extract agar and oatmeal agar, also, inoculums density and temperature on the spore carrying capacity and microcycle conidiation [3]. Previously, spore production of C. gloeosporioides was compared on solid media with liquid media [16]. C. gloeosporioides grow well on PDA (potato dextrose agar) and CWA (coconut watery endosperm) which contain appropriate amounts of carbohydrates, proteins, minerals and lipids [17]. The growth is completely inhibited at 10°C. Light is not necessary but enhance sporulation, pH 6 (for growth and sporulation) and germination is better on a more acidic medium. Czapek’s and yeast extract agar media give maximum growth [3].
The purpose of this present investigation was to determine in vitro the optimal growth of C. gloeosporioides using media, temperature, pH level and water potential (NaCl and KCl).
Methods
Fungal isolates
Four Colletotrichum gloeosporioides isolates were collected from leaves, peduncle and twigs from two Orange’s varieties Thomson and Malti orchards (Table 1). All isolates were purified and conserved in Plant Pathology laboratory for further uses.
C. gloeosporioides Isolates
Radial growth (cm/day)
Means
P values
Media
PDA
SDA
V8
Malt
MS
PARP
CgM1
0.759±0.03a*A**
0.758±0.03a
0.627±0.18ab
0.517±0.02cC
0.268±0.01d
0.000e
0.488
<0.01
CgS1
0.672±0.04aAB
0.762±0.03b
0.709±0.04c
0.583±0.03dB
0.293±0.02e
0.000f
0.503
<0.01
CgS3
0.567±0.21bcB
0.684±0.16ab
0.716±0.01a
0.464±0.08cD
0.334±0.05d
0.031±0.001e
0.466
<0.01
CgT1
0.622±0.14cB
0.759±0.04a
0.703±0.05ab
0.650±0.04bcA
0.290±0.02d
0.000e
0.504
<0.01
Means
0.655
0.741
0.689
0.554
0.296
0.008
P values
0.0006
0.095
0.0085
<0.01
0.06
0.186
Isolates
P<0.01
Media
P<0.05
Isolates X Media
P<0.01
Values of radial growth are the means of six replicates (three/experiment) ± standard error of the mean.
*Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of four Colletotrichum isolates for the same pH level.
**Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of eleven pH levels for the same Colletotrichum isolates.
Capital letters are for comparison of means in the same column.
Small letters are for comparison of means in the same row.
Table 1: Effect of media on mycelial growth of four Colletotrichum isolates at 25°C for 7 days.
Growth characters of C. gloeosporioides on different culture media
Six culture media were used to determine the most appropriate for the mycelial development of C. gloeosporioides. Those culture media were the PDA (Potato Dextrose Agar), SDA (Sabouraut Dextrose Agar), V8 juice medium, Malt, MS (Murashigeabd Skooge medium) and PARP (pimaricin + ampicillin + rifampicin + pentachloro-nitrobenzene agar). Mycelial plugs (8mm in diameter) obtained from the growing edge of colonies were transferred to the center of each culture medium and incubated at 25°C. There were two replicates for each isolate and medium combination. The diameter of each colony was measured twice perpendicularly when it reached at least two thirds of the plate and used to calculate the mean growth rate as cm per day.
Effect of temperature on mycelial growth
The mycelial growth of C. gloeosporioides was evaluated according to five temperatures (10, 15, 20, 25, 30 and 35°C) using PDA as medium. Mycelial plugs (8mm in diameter) obtained from the growing edge of colonies were transferred to the center of PDA plates and incubated in the dark at the experimental temperatures.
Effect of pH on mycelial growth
Eleven-pH level (5, 5.6, 6, 6.6, 7, 7.6, 8, 8.5, 9, 9.5 and 10) was evaluated on PDA medium. Mycelial plugs (8mm in diameter) obtained from the growing edge of colonies were transferred to the center of PDA plates which were adjusted to pH after the addition of 50mM citrate phosphate buffer (pH 4-7) or 50mM Trise HCl buffer (pH 8). Plates were incubated in the dark at 25°C. There were two replicates for each isolate and pH combination. Mean growth rates were evaluated as described before. The experiment was conducted twice in time.
Effect of water potential on mycelial growth
Different PDA plates amended with KCl or NaCl prior to sterilization to obtain seven water potential values: -0.34MPa (control: without KCl or NaCl), -0.5MPa, -1MPa, -2MPa, -3MPa, -4MPa, -5MPa and -6MPa [18-20]. Plates were incubated in the dark at 28°C. Mycelial plugs (8mm in diameter) obtained from the growing edge of colonies were transferred to the center of PDA plates.
Statistical analysis
Analyses of Variance (ANOVA) were conducted with data obtained from media, temperature, pH or water potential experiments to analyze potential trial and treatment interactions. In all cases, ANOVA analysis indicated that the data between the two repetitions were similar (P >0.05), thus data of all variables from both experiments were combined. Mycelial growth data were analyzed by multivariate factorial analysis using the GLM (SPSS.12). Means were compared with Waller-Duncan k-ratio t test.
Results
Growth characters of C. gloeosporioides on different culture media
The growth development of the four C. gloeosporioides isolates was determined after 7 days. The mycelial growth of the fungus (p<0.01) and the effect of culture media (p<0.05) differed significantly. Maximum radial growth of C. gloeosporioides was recorded on SDA medium (0.741cm/day), followed with V8 juice medium (0.689cm/day) followed by PDA (0.655cm/day). The lowest mycelial development was noted on PARP medium with values under 0.3cm/ day. Both CgS1 and CgT1 registered the highest radial growth value (0.5cm/day) (Table 1).
Effect of temperature on mycelial growth
The results of the temperature effect on mycelial growth of C. gloeosporioides showed that all isolates were able to grow on PDA at temperatures from 15 to 30°C. The temperature of 30°C was found to be significantly superior to other temperature levels by recording the maximum radial growth (0.773cm/day); At this temperature; CgS3 has recorded the highest radial growth with 0.858cm/day, in contrast of CgT1 which registered a similar value at 15°C (Table 2).
C. gloeosporioides
IsolatesRadial growth (cm/day)
Means
P values
Temperature °C
10
15
20
25
30
35
CgM1
0.000D**
0.75±0.02b*B
0.682±0.01aC
0.698±0.06bC
0.792±0.02bA
0.000D
0.487
<0.01
CgS1
0.000C
0.671±0.08cB
0.665±0.03aB
0.776±0.03aA
0.793±0.02bA
0.000C
0.484
<0.01
CgS3
0.000D
0.664±0.04cC
0.670±0.03aC
0.745±0.07abB
0.858±0.08aA
0.000B
0.489
<0.01
CgT1
0.000D
0.821±0.03aA
0.622±0.03bBC
0.6±0.06cC
0.65±0.04cB
0.000D
0.539
<0.01
Means
0.000
0.726
0.660
0.705
0.773
0.000
P values
<0.01
<0.01
<0.01
<0.01
Isolates
P<0.01
Temperature
P<0.01
Isolates X Temperature
P<0.01
Values of radial growth are the means of six replicates (three/experiment) ± standard error of the mean.
*Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of four Colletotrichum isolates for the same pH level.
**Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of eleven pH levels for the same Colletotrichum isolates.
Capital letters are for comparison of means in the same row.
Small letters are for comparison of means in the same column.
Table 2: Effect of temperature on mycelial growth of four Colletotrichum isolates for 7 days.
Effect of pH on mycelial growth
Radial growth on pH-adjusted PDA demonstrated a broad pH tolerance by C. gloeosporioides between 5 and 8. Optimal growth radial was recorded at 5 to 8 of 0.6cm/day; however, values were slightly less at the other pH level. The isolate CgT1 recorded the highest radial growth, and CgM1, the lowest one (Table 3).
C. gloeosporioides Isolates
Radial growth (cm/day)
Means
P values
pH Level
5
5.6
6
6.6
7
7.6
8
8.5
9
9.5
10
CgM1
0.625±0.01a*A**
0.505±0.07cB
0.608±0.03abA
0.533±0.06bB
0.595±0.02A
0.601±0.03aA
0.611±0.01aA
0.450±0.05cC
0.534±0.05bB
0.436±0.04cC
0.436±0.04cC
0.54
<0.01
CgS1
0.597±0.01bAB
0.549±0.02bDE
0.583±0.03bABCD
0.561±0.07abBCDE
0.601±0.03AB
0.604±0.01aA
0.586±0.02aABCD
0.555±0.05bCDE
0.563±0.04bBCDE
0.592±0.04abABC
0.529±0.07abE
0.57
<0.01
CgS3
0.582±0.04bA
0.566±0.03bAB
0.530±0.04cBCD
0.575±0.05abAB
0.577±0.06AB
0.489±0.1bD
0.549±0.03bAB
0.538±0.05bABC
0.544±0.02bABC
0.549±0.02bAB
0.497±0.02bCD
0.55
<0.01
CgT1
0.555±0.02cE
0.623±0.01aABC
0.631±0.01aAB
0.604±0.03aC
0.608±0.02BC
0.646±0.01aA
0.598±0.03aCD
0.609±0.04aBC
0.599±0.02aCD
0.575±0.03aDE
0.562±0.03aE
0.60
<0.01
Means
0.59
0.56
0.59
0.57
0.60
0.58
0.59
0.54
0.56
0.54
0.51
P values
<0.01
<0.01
<0.01
0.02
>0.05
<0.01
<0.01
<0.01
<0.01
<0.01
<0.01
Isolates
P<0.01
pH level
P<0.01
Isolates X pH level
P<0.01
Values of radial growth are the means of six replicates (three/experiment) ± standard error of the mean.
*Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of four Colletotrichum isolates for the same pH level.
**Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of eleven pH levels for the same Colletotrichum isolates.
Capital letters are for comparison of means in the same row.
Small letters are for comparison of means in the same column.
Table 3: Effect of pH level on mycelial growth of four Colletotrichum isolates at 25°C for 7 days.
Effect of water potential on mycelial growth
The effect of water potential on mycelial growth of the four C. gloeosporioides isolates is shown in Table 4 and Figure 1 for NaCl and Table 5 and Figure 2 for KCl, respectively. Similar results were obtained with both salt types, but, in general, C. gloeosporioides was more tolerant to KCl than NaCl, resulting in lower radial growth values when KCl was used to adjust water potential. Radial growth is positively correlated with water potential and was limited at -5 and -6.0 MPa. The highest values were recorded at -0.5MPa with 0.842 and 0.758 cm/day, for NaCl and KCl, respectively.
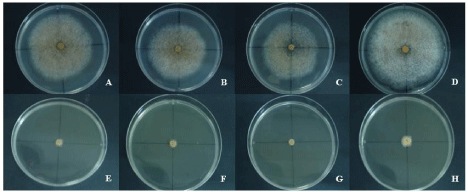
Figure 1: Mycelial growth of the four Colletotrichum isolates on PDA amended with NaCl and a water potential of -0.5MPa and of -6MPa; A and E: CgM1; B and
F: CgS1; C and G: CgS3; D and H: CgT1.
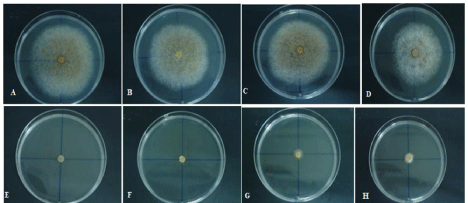
Figure 2: Mycelial growth of the four Colletotrichum isolates on PDA amended with KCl and a water potential of -0.5MPa and of -6MPa; A and E: CgM1; B and F:
CgS1; C and G: CgS3; D and H: CgT1.
C. gloeosporioides
IsolatesRadial growth (cm/day)
Means
P values
Na Cl
-0.34MPa (Control)
-0.5MPa
-1MPa
-2MPa
-3MPa
-4MPa
-5MPa
-6MPa
CgM1
0.905±0.04b*A**
0.856±0.05bB
0.826±0.05bB
0.545±0.06bC
0.455±0.04bD
0.352±0.04bE
0.257±0.02bF
0.192±0.02bG
0.549
<0.01
CgS1
0.881±0.05bB
0.792±0.05cB
0.721±0.05cC
0.543±0.02bD
0.425±0.04bcE
0.360±0.05bF
0.277±0.04abG
0.195±0.02bH
0.524
<0.01
CgS3
0.905±0.05bA
0.783±0.07cB
0.783±0.05bB
0.435±0.03cC
0.390±0.06cCD
0.348±0.05bD
0.281±0.02abE
0.191±0.02bF
0.515
<0.01
CgT1
0.952±0.01aA
0.936±0.02aAB
0.908±0.03aB
0.723±0.04aC
0.581±0.03aD
0.420±0.03aE
0.305±0.03aF
0.295±0.02aF
0.640
<0.01
Means
0.911
0.842
0.810
0.561
0.463
0.370
0.280
0.218
P values
0.001
<0.01
<0.01
<0.01
<0.01
<0.01
0.005
<0.01
Isolates
P<0.01
NaCl
P<0.01
Isolates X NaCl
P<0.01
Values of radial growth are the means of six replicates (three/experiment) ± standard error of the mean.
*Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of four Colletotrichum isolates for the same pH level.
**Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of eleven pH levels for the same Colletotrichum isolates.
Capital letters are for comparison of means in the same row.
Small letters are for comparison of means in the same column.
Table 4: Effect of water potential, established using NaCl, on mycelial growth of four Colletotrichum isolates at 25°C for 7 days.
C. gloeosporioides
IsolatesRadial growth (cm/day)
Means
P values
KCl
-0.34MPa (Control)
-0.5MPa
-1MPa
-2MPa
-3MPa
-4MPa
-5MPa
-6MPa
CgM1
0.747±0.04a*A**
0.733±0.02bA
0.506±0.02cB
0.520±0.03bB
0.419±0.02C
0.325±0.03abD
0.242±0.02E
0.146±0.01aF
0.455
<0.01
CgS1
0.696±0.04bA
0.714±0.03bA
0.603±0.05bB
0.548±0.04bC
0.426±0.04D
0.309±0.02bcE
0.256±0.04F
0.128±0.01cG
0.460
<0.01
CgS3
0.679±0.03bB
0.742±0.02bA
0.629±0.03abC
0.609±0.03aC
0.447±0.02D
0.339±0.02aE
0.238±0.02F
0.133±0.01bcG
0.477
<0.01
CgT1
0.755±0.02aB
0.843±0.04aA
0.647±0.05aC
0.608±0.05aC
0.444±0.02D
0.299±0.03cE
0.249±0.03F
0.141±0.02abG
0.499
<0.01
Means
0.719
0.758
0.596
0.574
0.434
0.318
0.246
0.137
P values
<0.01
<0.01
<0.01
<0.01
0.160
0.002
0.265
0.005
Isolates
P<0.01
KCl
P<0.01
Isolates X KCl
P<0.01
Values of radial growth are the means of six replicates (three/experiment) ± standard error of the mean.
*Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of four Colletotrichum isolates for the same pH level.
**Waller Duncan’s Multiple Range Test is for comparison of means among radial growth of eleven pH levels for the same Colletotrichum isolates.
Capital letters are for comparison of means in the same row.
Small letters are for comparison of means in the same column.
Table 5: Effect of water potential, established using KCl, on mycelial growth of four Colletotrichum isolates at 25°C for 7 days.
Discussion
The cultural studies of C. gloeosporioides on different solid media showed that SDA, V8 juice PDA media were the most favorable by the fungus. Many research revealed various response of this fungus to culture media. Sudhakar [21] reported that maximum radial growth recorded in five media, SDA, Richard’s agar, Brown’s agar, PDA and Oatmeal agar (OMA) did not differ significantly. Similarly, Zakaria [22] has reported that mycelial growth was noticeable on PDA, moderate on OMA and Cornmeal Agar (CMA). PDA and OMA were both revealed the most appropriate for development and sporulation of this pathogen [23]. In the other hand, Pandey et al. [24] revealed that Malt Extract Agar (MEA) showed to be appropriate for the development of this fungus. Likewise, Begam and Sharma [25], who has reported that the maximum growth of C. gloeosporioides was observed in Potato malt agar medium.
In this study, the four C. gloeosporioides isolates, showed optimal growth temperatures at 30°C. Sudhakar [21] found that pathogen could grow well at temperature of 20 to 30°C and relative humidity of 95%. Similar results were also reported by Estrada et al. [26]; Prasannakumar [27] and Prashanth [23]. The investigation of Sangeetha and Rawal [28] demonstrated significant differences between temperature and their interaction. They showed that the maximum colony diameter was observed at 28°C followed by 25°C, the temperature of 15°C showed the lowest mycelial growth. Tasiwal et al. [29] reported that 30°C is required for the good growth of C. gloeosporioides causal agent of anthracnose of papaya. Pandey et al. [24] found that the range of temperature 20-30°C was found optimum for the growth and sporulation by studying the optimum temperature for the growth C. gloeosporioides responsible of mango anthracnose. Similarly, Begam and Sharma [25] reported that the maximum growth of C. gloeosporioides causing disease in tealeaf (Camellia sinensis) was observed at 25 to 30°C.
The four C. gloeosporioides isolates radial growth on pH-adjusted PDA demonstrated a broad pH tolerance between 5 and 8. For the same goal, Kumara and Rawal [30] reported that C. gloeosporioides isolates grew well at pH 5 while sporulation was better at pH 6. Similarly, Thangamani et al. [31] who has studied the optimum pH indicated that the growth of C. musae was maximum at pH of 6.5-7. However, Deshmukh et al. [32] revealed that the fungus produced a maximum dry mycelial weight and sporulation at pH 5.5 and 6.5 in liquid media, respectively. Similarly, Begam and Sharma [25] reported that maximum growth was observed at pH 5.5.
Radial growth decreased progressively as water potential decreased and was limited at -5 and -6.0 MPa for both salts. No research has been done before to investigate the effect of osmotic and matric potentials on C. gloeosporioides. Our results showed that C. gloeosporioides could grow vegetative under low water potentials. This ability indicates the presence of adaptive mechanisms for life under variable environmental conditions. Adapting to a wide range of water potentials may be a strategy to exist as saprobe. Cervantes- Garcia et al. [33] observed a reduction in the pathogenicity of Macrophomina phaseolina on seeds of common beans, as NaCl concentrations increased in potato-glucose-agar medium. Dillard [34] reported that in water agar osmotically adjusted using either KCl or NaCl, maximum germination of conidia and growth from sclerotia of C. coccodes occurred at the highest osmotic potentials (-5 to -10 bars). Radial growth from sclerotia was less when KCl or NaCl amendments were used than when CaC12 or sucrose. Water potential is recognized as an important parameter in the ecology and growth of the pathogen [35]. The effects of water potential on growth determine the conditions under which pathogenesis in a host plant can occur. However, the influence of water potential on mycelial growth may differ with the nature of the medium [35]. In general, C. gloeosporioides required free water or relative humidity above 95 percent for conidial germination and appressorium formation [15].
This study provides further information on factors affecting growth of C. gloeosporioides under laboratory conditions. Thus, the results obtained here improve our understanding of the behaviour and growth of the pathogen, and can be useful to implement effective disease control.
References
- Observation Nationale de l’Agriculture. 2014.
- Centre Technique des Agrumes en Tunisie. 2014.
- Guarnaccia V, Groenewald JZ, Polizzi G, Crous PW. High species diversity in Colletotrichum associated with citrus diseases in Europe. Persoonia. 2017; 39: 32-50.
- Brown GE. Factors affecting post-harvest development of Colletotrichum gloeosporioides in citrus fruits. Phytopatho. 1975; 65: 404-409.
- Sutton BC. The genus Glomerella and its anamorph Colletotrichum. In: Bailey JA, Jeger MJ (eds), Colletotrichum: biology, pathology and control. CAB International, Wallingford, UK. 1992: 1-26.
- Freeman S, Shabi E. Cross-infection of subtropical and temperate fruits by Colletotrichum species from various hosts. Physio. And Mol. Plant Patho. 1996; 49: 395-404.
- Peng LJ, Yang YL, Hyde KD. Colletotrichum species on Citrus leaves in Guizhou and Yunnan provinces, China. Cryptogamie. Myco. 2012; 33: 267- 283.
- Damm U, Cannon PF, Woudenberg JHC. The Colletotrichum acutatum species complex. Studies in Myco. 2012a; 73: 37-113.
- Damm U, Cannon PF, Woudenberg JHC. The Colletotrichum boninense species complex. Studies in Myco. 2012b; 73: 1-36.
- Weir BS, Johnston PR, Damm U. The Colletotrichum gloeosporioides species complex. Studies in Myco. 2012; 73: 115-180.
- Aiello D, Carrieri R, Guarnaccia V. Characterization and pathogenicity of Colletotrichum gloeosporioides and C. karstii causing preharvest disease on Citrus sinensis in Italy. Journal of Phytopatho. 2015; 163: 168-177.
- Perrone G, Magista D, Ismail AM. First report of Colletotrichum kahawae subsp. ciggaro on mandarin in Italy. J. of Plant Patho. 2016; 98: 12.
- Rhaiem A, Taylor PW. Colletotrichum gloeosporioides associated with anthracnose symptoms on citrus, a new report for Tunisia. Eur. J. of Plant Patho. 2016; 146: 219-224.
- Ramos AP, Talhinhas P, Sreenivasaprasad S. Characterization of Colletotrichum gloeosporioides, as the main causal agent of citrus anthracnose, and C. karstii as species preferentially associated with lemon twig dieback in Portugal. Phytopara. 2016; 44: 549-561.
- Sharma M. and Kulshrestha S. Colletotrichum gloeosporioides: An Anthracnose Causing Pathogen of Fruits and Vegetables. Biosc. Biotechnol. Res. Asia. 2015; 12: 1233-1246.
- Slade SJ, Harris RF, Smith CS, Andrews JH. Microcycle conidiation and spore carrying capacity of C. gloeosporioides on solid media. App. and Env. Microbio. 1987; 53: 2106-2110.
- Santoso U, Kubo K, Ota T, Tadokoro T, Maekawa A. Nutrient composition of kopyor coconut (Cocos nucifera L.). Food Chem. 1996; 57: 299-304.
- Robinson RA, Stokes RH. Electrolyte Solutions, 2nd edn. Butterworth Scientific Publications, London, UK. 1959: 571.
- Armengol J, Alaniz S, Vicent A, Beltrán R, Abad-Campos P, Pérez-Sierra A, et al. Effect of dsRNA on growth rate and reproductive potential of Monosporascus cannonballus. Fungal bio. 2011; 115: 236-244.
- Ben Salem I, Boughalleb-M’Hamdi N, Souli M, Cherif M. Morphological and Biological Characteristics of Monosporascus cannonballus isolates, responsible of watermelon decline in the region of Kairouan. Res. in Plant Bio. 2011; 1: 28-37.
- Sudhakar. Biology and management of Stylosanthes anthracnose caused Colletotrichum gloeosporioides (Penz). Penz. And Sacc. M.Sc. (Agri.) Thesis, Uni. Agric. Sci., Dharwad (India). 2000: 34-35.
- Zakaria M. Morphology and cultural variation among Colletotrichum isolates obtained from tropical forest nurseries. J. of Trop. Forest Sc. 2000; 12: 1-20.
- Prashanth A. Investigation on anthracnose (Colletotrichum gloeosporioides (Penz.) Penz. And Sacc.) of pomegranate (Punica granatum L.). M.Sc. (Agri.) Thesis., Uni. Agric. Sci., Dharwad (India). 2007: 25-36.
- Pandey A, Yadava LP, Manoharan M, Chauhan UK, Pandey BK. Effectiveness of cultural parameters on the growth and sporulation of Colletotrichum gloeosporioides causing anthracnose disease of mango (Mangifera indica L.). OJBS. 2012; 12: 123-133.
- Begam J, Sharma GD. Comparative impact of physico-chemical and nutritional parameters on some phytopathogenic fungi isolate from the phyllosphere of diseased Tea leaf (Camellia sinensis L. O. Kuntze). Indian J. Appl. Res. 2015; 5: 235-238.
- Estrada AB, Dodd JC, Jeffries P. Effect of humidity and temperature on conidial germination and appressorium development of two Philippine isolates of the mango anthracnose pathogen Colletotrichum gloeosporioides. Pl. Pathol. 2000; 49: 608-618.
- Prasannakumar MK. Management of postharvest diseases of mango. M.Sc. (Agri.) Thesis, Uni. Agric. Sci., Dharwad (India). 2001.
- Sangeetha CG, Rawal RD. Temperature requirement of different isolates of Colletotrichum gloeosporioides isolated from mango. American-Eurasian J. Sci. Res. 2009; 4: 20-25.
- Tasiwal V, Benagi VI. Studies on the cultural and nutritional characteristics of Colletotrichum gloeosporioides, the causal organism of papaya anthracnose. Karnataka J. Agric. Sci. 2009; 22: 787-789.
- Kumara KLW, Rawal RD. Influence of carbon, nitrogen, temperature and pH on the growth and sporulation of some Indian isolates of Colletotrichum gloeosporioides causing anthracnose disease of papaya (Carrica papaya L.). Tro. Agr. Res. & Ext. 2008; 11: 7-12.
- Thangamani PR, Kuppusamy P, Peeran MF, Gandhi K, Raguchander M. Morphological and physiological characterization of Colletotrichum musae the causal organism of banana anthracnose. World J. Agric. Sci. 2011; 7: 743-754.
- Deshmukh AJ, Mehta BP, Sabalpara AN, Patil VA. In vitro effect of various nitrogen, carbon sources and pH regimes on the growth and sporulation of Colletotrichum gloeosporioides Penz. and Sacc. causing anthracnose of Indian bean. J. Biopest. 2012; 5: 46-49.
- Cervantes-Garcia D, Padilla-Ramirez JS, Simpson J, Mayek-Perez N. Osmotic potential effects on in vitro growth, morphology and pathogenicity of Macrophomina phaseolina. J. of Phytopatho. 2003; 151: 456-462.
- Dillard HR. Influence of temperature, pH, osmotic potential, and fungicide sensitivity on germination of conidia and growth from sclerotia of Colletotrichum coccodes in vitro. Phytopatho. 1988; 78: 1357-1361.
- Woods DM, Duniway JM. Some of effects of water potential on growth, turgor, and respiration of Phytophthora cryptogea and Fusarium moniliforme. Phytopatho. 1986; 76: 1248-1253.
