Research Article
Austin J Pathol Lab Med. 2014;1(2): 3.
Wild Rats as Reservoir of Trypanosoma Lewisi in Northwest India
Rayat CS* and Vasishta RK
Department of Histopathology, Postgraduate Institute of Medical Education and Research, India
*Corresponding author: Rayat CS, Department of Histopathology, Postgraduate Institute of Medical Education & Research, Chandigarh-160012, India
Received: September 01, 2014; Accepted: October 09, 2014; Published: October 13, 2014
Abstract
Trypanosomiasis is a drowsy condition caused by parasitization by extracellular hemoflagellate protozoa known as trypanosome. Pathogenic and nonpathogenic species of trypanosomes occur in a wide range of vertebrate hosts, ranging from fish to mammals. The host and vector happen together in the same habitat but their interaction could be influenced by feeding preferences of the vector due to variable climatic conditions. Trypanosoma lewisi is a natural hemoflagellate of rats (Rattus species) and other rodents in all areas of the world and considered to be nonpathogenic for human beings, however, in the recent past three reports of T. lewisi infection in infants had appeared form the coastal states located in the Southwest India. Anthropozoonosis has been well documented in trypanosomiasis and is a point of caution as animal reservoirs of a variety trypanosomes are around. Suppressed immune status of human hosts and changing feeding preferences of the vectors may complicate the scenario. A case report of Trypanosoma evansi infection in human adult from India could be considered as a flip in the vector behavior and pathogenicity of T. evansi, relatively due to over exposure to vectors and sub-optimal immune status of infected person. We could cite only single report about the detection of T. lewisi in the blood of wild rat (Rattus norvegicus) from India. Changing scenario of T. lewisi pathogenicity in infants, through the last few years in India, prompted us to conduct this study to detect the natural reservoir of T. lewisi in Northwest India. In this study we figure out the detection of T. lewisi infection in wild rats at Chandigarh; geographically located in northwest India.
Abbreviations
DNA: Deoxyribonucleic Acid; fps: Frames per Second; cm: Centimeter; μm: Micrometer; g: Gram
Introduction
Trypanosomiasis is popularly known as sleeping sickness. It is a somnolent condition, resulting from parasitization of blood by extracellular hemoflagellate protozoa known as trypanosome. This protozoan belongs to the order kinetoplastida and members of this order are recognized by the possession of kinetoplast, a DNA containing particle close to the flagellar basal body [1]. Pathogenic and nonpathogenic species of Trypanosoma occur in a wide range of vertebrate hosts, ranging from fish to mammals and are transmitted by blood sucking insects and other vectors [2].
Trypomastigote and epimastigote stages are common to nearly all trypanosome life cycles. There is considerable difference between the specific infections in different mammals and transmitting vectors [2]. Major species of the hemoflagellate pathogenic to man are: Trypanosoma gambiense and T. rhodesiense, which cause African sleeping sickness, and T. cruzi causing South American Trypanosomiasis or Chagas's disease. Trypanosoma lewisi is a nonpathogenic, natural blood parasite of the common black and brown rats in all parts of the world. In men the disease is transmitted by bites of the insect vector, tsetse fly (Glossina species), but T. lewisi is transmitted by rat fleas [2-4]. American trypanosomiasis can be transmitted to humans by blood sucking insects of the genra Tratoma, Rhodium and Panstrongylus [5]. Some trypanosomes of animals are pathogenic and cause morbidity and mortality in livestock. Trypanosoma brucei is found in wild game and domestic animals in tropical Africa. Trypanosoma evansi causes 'surra' in horses. Blood feeding tabanids are responsible for the transmission of T. evansi in horses and cattle. Trypanosoma vivax and T. congolense cause disease in domesticated and wild cattle and are transmitted by tsetse flies. Tsetse flies become infected by feeding on the blood of an infected man or animal. Trypanosomes taken up in blood meal by tsetse fly migrate to the cavity between peritrophic membrane and gut wall and multiply there. The life cycle in tsetse fly takes about three weeks. Trypanosoma lewisi is the natural parasite of rats (Rattus species) transmitted by rat fleas; by Nosopsyllus fasciatus in temperate regions and Xenopsylla cheopis in the warmer areas of the world [2]. Trypanosoma lewisi belongs to stercorarian group of trypanosomes and its trypomastigotes possess typically elongate slender body with pointed posterior end. Saxena and Miyata documented an unusual type of T. lewisi in the blood of Rattus norvegicus from New Delhi, India in 1993[6].
The aim of the present study was to screen the peripheral blood films of wild rats trapped around human dwellings at Chandigarh, India to detect the infection of T. lewisi to establish the existence of reservoir of this hemoflagellate parasite as we foresee an approaching danger of zoonotic infection to infants and immune suppressed patients in the light of most recent reports of T. lewisi infection in infants from the Southwest India [8-10]. Feeding preferences of vectors like Xenopsylla cheopis and tabanids have definitely changed in India as the reports of zoonotic infections are pouring in. Here we construe the evidence of the presence of T. lewisi infected rats at Chandigarh; geographically located in Northwest India. Our report could probably be an evidence of wild reservoir of Trypanosoma from this part of India and accentuate a need to be alert while investigating febrile patients with drowsiness. Similar studies by other researchers could be helpful to further establish the geographical migration of infected animals and possible risk of the disease to infants with poor innate immunity with/without primary immunodeficiency. Patients with acquired or therapeutic immunosuppression may also be considered at potent risk of zoonotic infections and investigated with alertness. There is a need to be extra vigilant while examining blood films from infants and immune compromised patients.
Materials and Methods
Three peripheral blood films each were prepared by tail prick of each of the ten wild rats trapped around human dwellings at Chandigarh, India during the last eight months. Giemsa stained peripheral blood films were screened microscopically using '40x' objective lens to detect any Trypomastigote of Trypanosoma species. Two of the ten rats were found to be positive for trypanosomes. Photomicrography was done using '100x' oil immersion lens. Olympus BX51 compound microscope (Olympus Corporation, Japan) was used for photomicrography and images were captured using Olympus DP20 Digital Camera (Olympus Corporation, Japan). Olympus DP20 is a 2 megapixel Camera with high color fidelity and transfers images at 15 fps. The camera control unit has been loaded with image capture software which displays images at 1600 x 1200 pixel size. Temporary image files were stored at 'Compact- Flash Memory Card' of the camera controller and downloaded to the attached PC. Images were accurately cropped without altering pixel values. Morphometry was done to determine the length of trypomastigotes through interactive procedure using Qwin-550 image analysis software (Leica Corporation, UK).
Results and Discussion
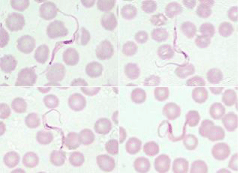
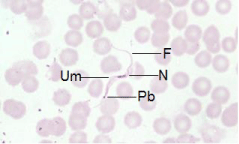
Two of the ten trapped wild rats weighing between 200 to 250 g were found positive for trypanosomes on thorough microscopic examination of Giemsa stained peripheral blood films. The composite photomicrograph presented as Figure 1, shows four extracellular hemoflagellate found in approximately 1 cm2 area of blood film towards tail. Essential morphological features of the hemoflagellate are well exhibited in Figure 2. Total length of trypomastigotes, including flagellum was measured to be 35-42 μm. The parasite could be T. lewisi as the species has already been documented to cause infection in wild rats and other rodents. However, molecular study was not done to establish the species of the trypanosome as the parasites were detected in its natural host. Extracellular hemoflagellate illustrated in the composite photomicrograph Figure 1 has slender bodies with oval shaped nuclei placed internally at anterior-posterior junction of the body. There were no granular inclusions in the parasites and the morphological features conform to the features of T. lewisi, as documented in literature by various researchers [1-6].
Figure 1: Composite photomicrograph showing four trypomastigotes of Trypanosoma lewisi as detected in a peripheral blood film from a brown wild rat (Giemsa stain, 1000x).
Figure 2: Photomicrograph of a trypomastigote of Trypanosoma lewisi exhibiting F: Flagellum; N: Nucleus; Um: Undulating Membrane and PB: Parabasal Body of kinetoplast. (Giemsa stain, 1000x).
Trypanosoma lewisi has long been considered nonpathogenic natural hemoflagellate of black and brown wild rats and is transmitted by rat fleas [2-4]. Host and vector interactions cannot be assured as climatic changes may influence the vector behavior. Anthropozoonosis has been well documented in trypanosomiasis. In the year 2006 a case of human trypanosomiasis caused by T. evansi, confirmed by optical, serological and molecular-biological methods, was reported from rural area of Chandrapur district of Maharashtra state of India [7]. Trypanosoma evansi is known to cause severe disease in camels, horses, dogs and the Indian elephant and produces benign infection in cattle, water buffaloes, pigs, sheep, goats and donkeys, which act as reservoir hosts [2]. During the period spreading from 2007 to 2012, three cases of febrile illness caused by T. lewisi parasite had been documented in infants from India [8-10].
Nothing seems certain in medical science and anthropozoonosis is a cause of concern in trypanosomiasis and malaria too. Plasmodium knowlesi, a zoonotic malarial parasite has become a major cause of human malaria in Southeast Asia [11]. Naturally or therapeutically immune suppressed patients may fall prey to anthropozoonosis to any extent. Essential immunosuppression for immune compromisation for organ survival in organ transplantation cases may make the patients susceptible to unpredictable zoonotic infections. Wild rats are established reservoirs of T. lewisi and changing feeding preferences of existing vectors or new generation of vectors as well as immune status of human beings may tilt the scenario anytime as the effect of compounding factors like global warming and pollution are unpredictable. Our study suggests that a considerable population of wild rats is positive for T. lewisi in the Northwest India. The morphological microscopic features of trypomastigotes reported by us are consistent with those documented by Saxena & Miyata [6]. Millaco et al. (2013) and Pumhom et al. (2014) have documented the prevalence of T. evansi and T. lewisi in rodents and species of rats trapped form around the human dwellings in Southeast Asia [12,13]. The Indian subcontinent is considered free of human trypanosomiasis but there could be a need to be extra alert while examining blood films from patients with 'pyrexia of unknown origin' (PUO) and get investigated our pets and domesticated cattle for trypanosomes.
Conclusion
Biomedical scientists and clinicians have been playing a vital role to save the mankind from vector borne diseases. Nutrition, temperature and hydrogen ion concentration (pH value) are three major factors for the growth and survival of protozoal parasites in any host. We have been burning millions of liters of petroleum products daily and polluting the environment with residual hydrocarbons and too have pricked the outer space with rockets resulting in global warming. Changing climatic conditions have been offsetting the established notions about the pathogenicity of Trypanosoma species and feeding preferences of vectors. It has been established with molecular studies by Pumhom and associates that the prevalence of T. lewisi is very high in the species of rats trapped around human dwellings in Southeast Asia [13]. Naturally or therapeutically immune suppressed patients may fall prey to zoonotic infection of Trypanosoma species as vector behavior is never certain. Trypanosoma lewisi infected rats are all around with rat fleas (vectors) and human susceptibility to zoonotic infections seems uncertain under the changing climatic conditions and congesting world due to traveling, migration of human population & animals and transportation of goods. Alertness of clinicians, hematopathologists, parasitologists and basic medical scientists is must while investigating febrile patients with pyrexia of unknown origin to offset the risk of unknown zoonotic infections. Surveillance studies at microscopic and molecular level are very much needed to establish the prevalence of vector borne parasitic infections in wild rodents, pets and domesticated cattle.
References
- Vickerman K. The diversity of the kinetoplastid flagellates. Lumsden WHR, Evans DA, editors. In: Biology of the Kinetoplastida. New York: Academic Press. 1976; 1: 1-34.
- Kettle DS. Medical and Veterinary Entomology. United Kingdom: CAB International. 1993; 563-587.
- Baker JR. 3rd Symposium of British Society of Parasitologists. Taylor AER, editor. In: Evolution of Parasites. Taylor Oxford: Blackwell Scientific Publishers. 1965.
- Chatterjee KD. Parasitology (Protozoology and Helminthology) 12th edn. India: Chatterjee Medical Publishers. 1980.
- Nogueira N, Coura JR. In: Tropical and Geographical Medicine. Warren KS, Mahmoud AAF, editors. New York: McGraw-Hill Book Company, 1984; 253-69.
- Saxena VK, Miyata A. An unusual morphological type of Trypanosoma (Herpetosoma) lewisi (Kent, 1880) detected in the blood of Rattus norvegicus in India. J Commun Dis. 1993; 25: 15-17.
- Powar RM, Shegokar VR, Joshi PP, Dani VS, Tankhiwale NS, Truc P, et al. A rare case of human trypanosomiasis caused by Trypanosoma evansi. Indian J Med Microbiol. 2006; 24: 72-74.
- Kaur R, Gupta VK, Dhariwal AC, Jain DC, Shiv L. A rare case of trypanosomiasis in a two month old infant in Mumbai, India. J Commun Dis. 2007; 39: 71-74.
- Shah I, Ali US, Andankar P, Joshi RR. Trypanosomiasis in an infant from India. J Vector Borne Dis. 2011; 48: 122-123.
- Verma A, Manchanda S, Kumar N, Sharma A, Goel M, Banerjee PS, et al. Trypanosoma lewisi or T. lewisi-like infection in a 37-day-old Indian infant. Am J Trop Med Hyg. 2011; 85: 221-224.
- Parija SC, Giri S. Emerging protozoal pathogens in India: How prepared are we to face the threat? Trop Parasitol. 2012; 2: 13-19.
- Milocco C, Kamyingkird K, Desquesnes M, Jittapalapong S, Herbreteau V, Chaval Y, et al. Molecular demonstration of Trypanosoma evansi and Trypanosoma lewisi DNA in wild rodents from Cambodia, Lao PDR and Thailand. Transbound Emerg Dis. 2013; 60: 17-26.
- Pumhom P, Pognon D, Yangtara S, Thaprathorn N, Milocco C, Douangboupha B, et al. Molecular prevalence of Trypanosoma spp. in wild rodents of Southeast Asia: influence of human settlement habitat. Epidemiol Infect. 2014; 142: 1221-1230.