
Review Article
Austin J Pathol Lab Med. 2019; 6(1): 1025.
Mathematical Krause-Hegselmann (KH) Model Predicts Chemotherapeutic Resistance to Cisplatin in Human Cervical Cancer Cell Lines
Chen JZ1, Wu R2, Simpkins H2, Sun Z3 and Chen J2*
1Jericho High Senior School, Jericho, New York
2R.W, H.S., J.C., Feinstein Institute for Medical Research, Northwell Health, Pathology and Laboratory Medicine, Staten Island University Hospital, Staten Island, New York 10305
3Z.S., Academy of Math. & Systems Science, Chinese Academy of Sciences, New York
*Corresponding author: Chen J*, Department of Pathology and Laboratory Medicine Staten Island University Hospital, Northwell Health, Staten Island, New York 10305
Received: August 24, 2019; Accepted: September 17, 2019; Published: September 24, 2019
Abstract
Objective: Solid tumor usually has a complex cellular components and intercellular interaction in a complex tumor microenvironment. We use a mathematical KH model to predict the change of drug resistance to cisplatin in a mixed sensitive and resistant cancer cell population and then use cytosensitivity assay to evaluate if our model can accurately predict this change predicted by KH model.
Methods and Materials: We used the KH model to predict the final increasing resistance in mixed cell population. To confirm the prediction, we focused on a mixed cell population to investigate the change of resistance to cisplatin. We mixed the sensitive and resistant cancer cells and studied their resistance to cisplatin. At the same time, separate sensitive and resistant cells are set up as controls. Our hypothesis is that after mixing, due to intercellular connection and communication (“talking” &“opinion change”), the resistant cells will instigate the sensitive cells to become more resistant, and therefore, the mixed population will show a different property with more resistance. Two human cervical cancer cells 2008 (sensitive) and 2008/C13*(resistant) were grown in RPMI 1640 medium supplemented with 10% FBS. The cytosensitivity was evaluated with MTT assay.
Results: We mixed same amounts of sensitive and resistant cancer cells and treated them with cisplatin and compared with their IC50s of parental cells. In groups of 1000, 2000, 4000, and 6000 cells, the ratios of the mixed cell population IC50s over the average IC50s of sensitive and resistant cells, showed the same tendency, namely, with the longer incubation period from day 2 to day 7, the ratios were higher, which meant the mixed cell population got relatively higher resistance to cisplatin. In other words, after mixing the cells, the sensitivity of the mixed cells were not as simple as the average of sensitive and resistant cells, but showing more resistance, which is interestingly compatible with our model prediction.
Conclusion: For the first time, with a KH model we correctly predicted the increased drug resistance in a mixed cancer cell population, which was demonstrated by our in vitro experiment. Based on the finding above, we see that the mathematical KH model can be employed to supply with novel idea in the research of chemoresistance field. The underlying mechanism is open for further exploration.
Keywords: Krause-Hegselmann (KH) model; Chemotherapy; Cisplatin; Drug resistance; Human cervical carcinoma
Introduction
Chemotherapeutic resistance, no matter whether intrinsic or acquired, is a leading obstacle to successful management of patients with cancer. The majority of the current research in chemoresistance field are mainly focusing on comparing resistant cells with sensitive ones, finding out the difference, focusing on the difference to elucidate the resistance mechanisms and then try to figure out a way to reverse them. An irregularly shaped solid tumor, actually is not composed of pure cell population but a cellular mixture from single or multiple clones of stem cells [1], which are characterized with different biological properties including different resistance to chemotherapeutic drugs. Plus the complex tumor microenvironment due to different degree of hypoxia, glucose level, and blood supply [2], the fact that a solid tumor is composed of a mixture of tumor cells with different resistant degree is easily understandable. Thus, how do these different cell populations affect each other in a solid tumor? Are the more resistant cells making the sensitive ones more resistant? If the answer is yes, the current management for patients with solid cancer may need to be retuned. For example, during the current chemotherapy cycles of ovarian cancer, the regimen may not be able to keep same from cycle one to cycle six, but should be retuned according to the profile of biological changes, such as increasing resistance, to further improve patient prognosis. Based on the changing biological properties of a solid tumor with cellular heterogeneity, this kind of adjustment will act in concert with the initial intention of promoting wellness in current precision medicine.
KH model is a simple model often utilized in opinion dynamics to predict the opinion evolution on any given issue of a population. We speculate the tumor cells in a solid tumor would behave like a community and these cells will interact as do people. Based on KH model, our hypothesis is that after mixing the sensitive and resistant cells, due to intercellular connection and communication, the resistant cells will instigate the sensitive cells to become more resistant and not the reverse on drug exposure pressure, and therefore, the mixed population will show a different property from the original status – namely, more resistance, which we call it “1+1>2 effect”. This study is to focus on a mixed cell population to investigate the change of resistance to cisplatin, which is the most commonly used therapeutic drug in chemotherapy. To our knowledge, this is the first paper to use a mathematical KH model to predict and investigate the biologic change.
Methods and Materials
Krause-Hegselmann (KH) model and Prediction for drug resistance
As the picture showed in Figure 1a, each dot in the KH model stands for an individual with different opinion on any type of different topics like entertainment, faith, sport, etc. The KH model is most often used to predict evolution in opinions of persons. Opinions are quantified as numbers between 0 and 1, inclusive, based on extremeness of stance, although the range has no significance and can be changed. Opinions of individuals respectively, on any given issue, as a function of time, will change as a result of interaction and influence. A confidence bound ? is given so that agent will interact/influence each other only if . Each agent’s opinion is updated as follows:
Simply speaking, as the KH model in the figure showed Figure 1, in a community with many individuals with different opinions (a), when the confidence bound (?) from b to f increases enough, the opinion of this same individual group (population) will finalize into one, meaning reaching the same opinion on a single topic finally. This model can be taylored to predict changes in cell resistance, where each cell takes the place of an individual, with “opinions” (sensitive or resistant) represented by their sensitivity to cisplatin.
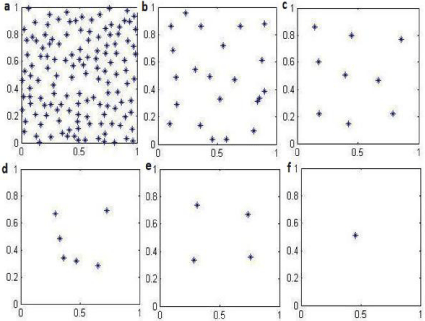
Figure 1: KH typical model in a community with many individuals with
different opinions (a), when the confidence bound (ε) from (b) to (f) increases
enough, the opinion of this individual population will finalize into one, meaning
finally reaching the same opinion on one single topic.
Cell lines
The human cervical cancer cells 2008 (cisplatin sensitive) and C13 (cisplatin resistant) were grown in RPMI 1640 medium supplemented with 10% FBS and gentamycin at a final concentration of 10 μg/ml as described previously. Cell culture reagents and Gentamicin were obtained from Cell-grow (Herndon, VA). The drug cisplatin was from Aldrich Chemical Co. (Milwaukee, WI).
Cytosensitivity test
Measurement of cell viability and proliferation forms the basis for numerous in vitro assays of a cell population’s response to chemotherapeutic drug. The cytosensitivity to cisplatin of the mixed cell group was assessed and compared to the parental cisplatin-sensitive and -resistant cells utilizing IC50 values, which were determined by the 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assay. The reduction of tetrazolium salts is now widely accepted as a reliable way to examine cell proliferation. The yellow tetrazolium MTT (3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide) is reduced by metabolically active cells, in part by the action of dehydrogenase enzymes, to generate reducing equivalents such as NADH and NADPH. The resulting intracellular purple formazan can be solubilized and quantified by spectrophotometric means. The MTT Cell Proliferation Assay measures the cell proliferation rate and conversely, when metabolic events lead to apoptosis or necrosis, the reduction in cell viability. The number of assay steps has been minimized as much as possible to expedite sample processing. The MTT Reagent yields low background absorbance values in the absence of cells. For each cell type the linear relationship between cell number and signal produced is established, thus allowing an accurate quantification of changes in the rate of cell proliferation.
MTT assays were performed as described previously. Briefly, three group of cells, including the sensitive 2008 cells (called sensitive group), the resistant C13 cells (called resistant group), and the mixed sensitive and resistant cells (called mixed group), were seeded onto 96-well plates in triplicate at different cell number (1000, 2000, 4000, and 6000 cells) with different concentrations of cisplatin (0, 0.1, 0.5, 1, 2, 5, 10, and 20 µM). The mixed cell group is composed of final equal cell number but with half amount of sensitive and resistant cells (namely 500/500, 1000/1000, 2000/2000, 3000/3000) to keep the total cell number unchanged. Sensitive group and resistant group were set up at the same time as controls. The cells were incubated for 1, 2, 3, 4, 5, 6, and 7 days at 37 °C in tissue culture incubator with 5% of carbon dioxide (CO2). 10 μl of MTT (stock concentration 5 mg/ml) was added to each well 5 hours before the end of each incubation period. The resulting intracellular purple formazan was solubilized in 100 μl of isopropanol/hydrochloride (v:v=100:1). The plates were then scanned at 595 nm in a 96-well plate reader. Each experiment was performed three times separately in triplicate.
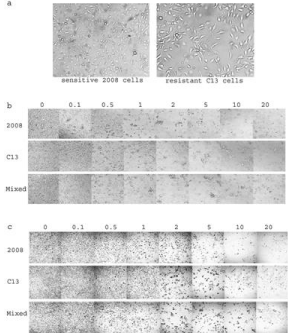
Figure 2: Human cervical cancer sensitive (2008) and resistant (2008/C13*)
cell morphology (a) and toxicity (b) and formazan crystal formation with MTT
(c) at different concentrations of cisplatin.
Statistical analysis
The linear regression analysis for IC50s and paired t-test were performed using Excel and the SigmaStat Statistical Analysis System, Version 1.01. P values were considered to be significant when p <0.05.
Results
Mathematical KH model prediction
Based on the simple observation, we replaced each individual in a community with a single cancer cell and the sensitivity to cisplatin (sensitive or resistant) as two different opinions in a mixed cell group with sensitive and resistant cancer cells. Then we treated the mixed cell group and checked their change of sensitivity from day 2 to day 7. Based on the same mechanism of KH model opinion dynamics, our hypothesis was that under the pressure of chemotherapeutic drug treatment, the final confidence bound (?) should be big enough as “survival”, and therefore, the final “opinion” of the mixed cell group should be more resistance, other than the reverse.
Experimental results
To demonstrate the novel thought above, we employed MTT assay. With MTT assay in our laboratory, we usually treated cells for 72 hours because the doubling times for both 2008 and C13 cells are 24 hours [3]. In this experiment, we did not collect data after 24-hour treatment (day 1) based on our previous experience because without 2-3 doubling time, it is difficult to evaluate the effect of the drug on cells. The IC50s at short cutoff time will be dramatically high as the data shown here at Day 2. For chemosensitivity evaluation, we even did not treat cells more than 3 days. However, to evaluate the inhibitory effect by cisplatin, MTT assay is still a good approach for a short or longer time as long as living cells exist (Figure 2). The IC50s to cisplatin at Day 3 for 2008 (0.99±0.23µM) and C13 (6.60±1.03µM) cells are consistent with the data in our previous report, meaning that C13 (a.k.a 2008/C13*5.25) cells are about 7 times more resistant to cisplatin than the parental sensitive 2008 cells [4].
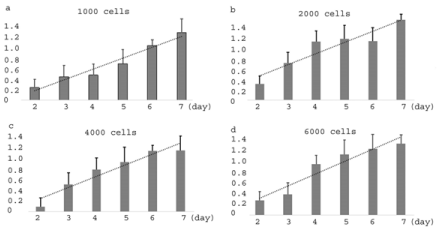
Figure 3: The increasing ratios of mixed cell group IC50s over the average
IC50s of sensitive and resistant cells in 1000(a), 2000(b), 4000(c), and
6000(d) cells.
From day 2 through day 7, the MTT assay results suggested decreasing IC50s to cisplatin (Table 1, the representative 4000-cell group, which shows the same tendency as other cell groups with 1000, 2000, and 6000 cells(data not shown here) in sensitive 2008 group, resistant C13 group, and mixed cell group. Particularly, by comparison with the average IC50s, the mixed cell group did not show increased IC50s values but lower from day 2 through day 5 and insignificant change on day 6 and day 7. By the values, the IC50s of mixed group are only a little bit higher than those of sensitive group (2008 cells) and between sensitive group and resistant group. These findings are consistent in every different cell groups (1000, 2000, 4000, and 6000 cells). However, when we calculated the ratio of the IC50s of mixed groups to the IC50s of the average, we surprisingly found these ratios are beautifully increasing in every cell groups from day 2 through day 7 Figure 3a,b,c,d. This finding simply means that even though all IC50s of three groups are decreasing in parallel, the IC50s of mixed cell group are dropping relatively slowly when compared with the average IC50s of parental cells. In other words, these mixed cells are not just mixed together as two independent cellular populations, but even more, they affected each other by showing a more resistant tendency to cisplatin from day 2 through day 7. This is a very interesting finding that we have not seen being reported before. And more important, the model accurately first predicted this phenomenon, which was later on confirmed here with a simple cytosensitivity assay.
IC50 values to cisplatin ( mean ± S.D.)
Cell group
2008
C13
mixed cells
average IC50(2008 and C13)
Ratio of IC50s of (mixed group/average) (%)
Day 2
11.88±3.54
768.36±23.65
32.52±5.66*
390.12±18.45
0.08
Day 3
0.99±0.23
6.60±1.03
1.74±0.34*
3.80±0.78
0.46
Day 4
0.50±0.12
1.04±0.35
0.55±0.23*
0.77±0.24
0.71
Day 5
0.44±0.09
0.63±0.11
0.45±0.24*
0.54±0.17
0.84
Day 6
0.33±0.13
0.54±0.07
0.44±0.17#
0.43±0.11
1.03
Day 7
0.28±0.07
0.42±0.09
0.36±0.10#
0.35±0.08
1.04
Table 1: Cytosensitivity to cisplatin in 4000-cell group evaluated by MTT assay. The values presented from three independent experiments, each performed in triplicate. These values stand for IC50 values to cisplatin in each cell group. Data are expressed as mean ±S.D.. *p<0.05 but #p>0.05 when compared with average IC50s.
Discussion
In this study we employed a mathematical KH model to predict the possible dynamic change of cisplatin resistance in a mixed cell group with sensitive and resistant human cervical cancer cells. In the KH model, we assumed the confidence bound parameter ? as survival, it predicted that the mixed cell group would be more resistant to cisplatin as long as these cells are mixed together for enough long period. Based on this novel thought, we did cytosensitivity assay by mixing both sensitive and resistant cervical cancer cells. Our findings in all cell number groups showed beautiful consistency, namely, the increased IC50s or resistance to cisplatin when compared with corresponding average IC50s from day 2 through day 7. To our knowledge, this is the first paper that employed a mathematical KH model to predict and confirmed the biological change of increased drug resistance in a mixed cell population. The importance that matters is the mixed cell population got a new changed biological characteristic- more resistance! The mixed cells are not simply admixed but a real mixture with more resistant change, which would imply the more resistant feature in a solid tumor with mixed cellular populations.
In a real solid tumor environment, the intercellular interactions are very profound. As a matter of fact, a solid tumor consists not only of a heterogeneous population of cancer cells, but also a variety of secondary or resident entrapped host connective tissue cells, secreted cytokine factors and Extracellular Matrix (ECM) proteins, collectively known as the tumor microenvironment. Many studies have reported that tumor microenvironment acts as a mechanism of resistance to chemotherapy [5,6]. As part of this, the intercellular interaction particularly between sensitive and resistant cells is not clear. Similarly, 35 years ago, Tofilon et al, [7] grew multicellular spheroids from mixtures of rat brain tumor sensitive (9L) cells and resistant (R3) cells. They found the percentages of each cell subpopulation in these spheroids were approximately the same as those used to initiate spheroids and the sensitivity of 9L cells to BCNU in mixed-cell spheroids was decreased as the percentage of R3 cells increased. They thought these effects were probably the result of an interaction between the two cell subpopulations held in 3-D contact. Recently Kaznatcheev et al, [8] developed a “game assay” to measure effective evolutionary games in co-cultures of non-small cell lung cancer cells that are sensitive and resistant to the anaplastic lymphoma kinase inhibitor alectinib. They tried to treat the “player” (cancer cells) and also” the game” (the interactions by other cells like fibroblasts). As part of mechanisms of microenvironmental associated drug resistance, various cytokines and growth factors from tumor cells or stromal cells play an important role. A recent study [9] demonstrated that, in response to targeted therapy with BRAF inhibition in melanoma, drug-sensitive cancer cells can secrete various cytokines including IGF, HGF, etc, into the microenvironment. These factors can in turn activate the survival signaling of drug-sensitive cells and can promote the proliferation, migration and metastasis of drug-resistant cancer cells. Based on our previous research experience on multicellular aggregates [10-13] and our current findings here, we speculate that under the treatment pressure of therapeutic drugs, the intercellular communication between different cell populations, for example, sensitive cells and resistant cells, should not be a one-way reaction, like the findings above in which cytokines from sensitive cells finally promoted the actions of resistant cells, but an interaction among different cell populations with a common purpose – survival! The investigation of mechanism behind this increased resistance is underway in our lab.
In summary, we can clearly see here that the mathematical KH model offered us a novel idea that could predict the biological change of more resistance to cisplatin in the mixed cancer cell groups. In the field of tumor drug resistance, a numerous experimental results and a mass of high-throughput data have been accumulated. However, resistance is still a main challenge to successful management in current clinic. Novel thoughts, hypotheses, or strategies, like the one here with a mathematical KH model, are more than welcomed to be put forward for further study in the future.
Funding
Dr. Henry Simpkins’ Lab, Feinstein Institutes for Medical Research, Northwell Health, NY, supported the work.
Availability of Data and Materials
The corresponding author (jchen3@northwell.edu) on reasonable request can provide raw data of results.
Authors' Contributions
John C. conceived the experiments, H.S. read and revised the manuscript. RW, J C wrote the
manuscript, ZS guided on KH model, John, JC designed and performed the experiments. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Acknowledgements
Thanks to Dr. Henry Simpkins and Dr. Wu’s guiding once in experiments.
References
- Egeblad M, Nakasone ES, Werb Z. Tumors as organs complex tissues that interface with the entire organism. Dev Cell. 2010; 6: 884-901.
- Jiang E, Yan T, Xu Z, Shang Z. Tumor Microenvironment and Cell Fusion. Biomed Res Int. 2019.
- Montopoli M, Ragazzi E, Froldi G, Caparrotta L. Cell-cycle inhibition and apoptosis induced by curcumin and cisplatin or oxaliplatin in human ovarian carcinoma cells. Cell Proliferation. 2009; 2: 195-206.
- Chen J, Adikari M, Pallai R, Parekh HK, Simpkins H. Dihydrodiol dehydrogenases regulate the generation of reactive oxygen species and the development of cisplatin resistance in human ovarian carcinoma cells. Cancer Chemother Pharmacol. 2008; 6: 979-987.
- Gonçalves-Ribeiro S, Díaz-Maroto NG, Berdiel-Acer M, Soriano A, Guardiola J, Martínez-Villacampa M, et al. Carcinoma-associated fibroblasts affect sensitivity to oxaliplatin and 5FU in colorectal cancer cells. Oncotarget. 2016; 37: 59766-59780.
- Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Letter. 2017; 387: 61-68.
- Tofilon PJ, Buckley N, Deen DF. Effect of cell-cell interactions on drug sensitivity and growth of drug-sensitive and -resistant tumor cells in spheroids. Science. 1984; 4676: 862-864.
- Kaznatcheev A, Peacock J, Basanta D, Marusyk A, Scott JG. Fibroblasts and alectinib switch the evolutionary games played by non-small cell lung cancer. Nat Ecol Evol. 2019; 3: 450-456.
- Obnauf AC, Zou Y, Ji AL, Vanharanta S, Shu W, Shi H, et al. Therapy-induced tumor secretomes promote resistance and tumor progression. Nature, 2015; 520: 368-372.
- Liu Z, Chen J, Wan XP. The adhesion and migration of SKOV3 spheroids in vitro. Progress in Obstetrics and Gynecology. 2007; 5: 341-344.
- Liu ZF, Chen J, Wan XP. Ovarian carcinoma SKOV3 spheroid adhesion to the Extracellular Matrix (ECM) and live human mesothelial cells, and assessment of the role of β1-integrin in mediating this process. Chin J of Cancer Prevention and Treatment. 2006; 14: 1050-1053.
- Chen J, Gu KJ, Wan XP. Gap Junction Intracellular communication and ovarian cancer drug resistance. Chin Journal of Clinical Medicine. 2003; 2: 129-132.
- Xi XW, Chen J, Feng YJ, Wan XP. Differential expression of CD44, CD29, CD54 and E-cadherin in human ovarian cancer SkOV-3ip1 multicellular aggregates. Chin J of Cancer Research. 2003; 1: 19-23.