
Research Article
J Pediatri Endocrinol. 2017; 2(2): 1018.
Pattern of Type I Diabetes Mellitus in Yemeni Children
Ba-Saddik IA* and Aghbari NA*
Department of Paediatrics, Faculty of Medicine and Health Sciences, Aden University, Yemen
*Corresponding author: Ba-Saddik IA, Paediatric and Child Health Department, Faculty of Medicine & Health Sciences, University of Aden, Yemen
Received: August 19, 2017; Accepted: November 24, 2017; Published: December 01, 2017
Abstract
Type 1 diabetes mellitus is the most frequent endocrine- metabolic disorder of childhood and adolescents. It is an important public stressful current issue because of its long term adverse complications.
Objectives: To describe the clinical pattern, management and outcome of diabetes mellitus among Yemeni children in Aden.
Patients and Methods: All admitted children holding the diagnosis of Type 1 diabetes mellitus with or without diabetic ketoacidosis were included in this study during January1999 to December 2001 at Al–Sadaka paediatric teaching hospital, Aden, Yemen
Results: A total of 55 patients were admitted during the study period with sex variation of male to female ratio of 1.5:1. The mean age (SD) was 10.5 (3.2) and the peak age group was 10-14 years in 61.8% of the patients with the youngest male infant of 16 months. A positive family history was obtained in 9 patients. Under nutrition was evident significantly in 80% of our patients. The major complaints of polydepsia and polyuria were present in 72.2% of the patients. The mean (SD) hospital stay was 13.6 (7.3) days. All patients were treated with insulin. Diabetic ketoacidosis occurred in 11 patients of whom two unfortunately died.
Conclusion’s and Recommendations: Type 1 diabetes mellitus is a major health problem among Yemeni children. It is essential to establish a diabetic registry in Yemen and stress on importance of carrying out an epidemiological evaluation on type 1 diabetes mellitus. We recommend the provision of best health care services which should be available and affordable to every diabetic child.
Keywords: Type I Diabetes Mellitus; Ploydepsia polyuria; Diabetic Ketoacidosis; Insulin
Introduction
Type 1 Diabetes Mellitus (T1DM) is the most prevalent chronic endocrine-metabolic disorder of childhood and adolescence characterized by hyperglycemia which maybe acute and sometimes life-threatening [1]. T1DM can occur at any age and is characterized by the marked and progressive inability of the pancreas to secrete insulin because of autoimmune destruction of the beta cells [2,3].
In 1997 the American Diabetes Association and WHO recommended altering the classification to define four main subtypes of Diabetes Mellitus (DM) [2]. T1DM includes immune mediated and idiopathic forms of β cells dysfunction which lead to absolute insulin deficiency. Type 2 Diabetes is a disease of adult onset which may originate from insulin resistance and relative insulin deficiency or from a secretory defect. Type 3 Diabetes covers a wide range of specific types of diabetes including the various genetic defect of β cells function, genetic defect in insulin action and disease of the exocrine pancreas. Type 4 Diabetes is gestational diabetes [2,4].
T1DM accounts for 5 – 10% of diabetes that usually occurs in children or young adults [3]. The peak incidence is during puberty and there is no sex predominance [1,4]. The seasonal variation peak onset is during the fall and winter [5]. Internationally rates of T1DM are increasing [6,7]. In Europe, the Middle East, and Australia, rates of T1DM are increasing by 2-5% per year [5,6,7]. Globally the incidence and prevalence of T1DM is highly variable among different Arab developing and developed countries [5,6]. A recent published review of the Nile Delta region in Egyptian children 0 to 18 years of age reported the prevalence of TIDM was 1.9, 15.5 and 26.8/100,000 in the years of 1996, 2006 and 2011 respectively [8]. In Iraq the estimated prevalence increased from 7.8 in 1995 to 14.2 in 2000 and to 24.7 in 2014 per 100 000 under 15 years old children [9]. In Eastern province of Saudi Arabia the incidence has doubled from 18.1 per 100,000 to 36.9 per 100,000 per year between 1990 and 2007 [10], while in Kuwait native children, the incidence increased from 17.7 per 100,000 per year (1992 to 1994) to 40.9 per 100,000 per year (2011 to 2013) [11].
Multiple factors are involved in the etiology of T1DM including genetic related to Human Leukocyte Antigen System (HLAS), environmental factors mainly viruses and immunological reactions [12,13]. The triggering factors are viral infections, early introduction of cow’s milk, non breast fed infants, antecedent stress, and exposure to certain chemical toxins [14,15].
In T1DM, 97% of patients present with the classical symptoms of polyuria, polydepsia, and polyphagia and weight loss. 10 – 20% initially present with frank Diabetic Ketoacidosis (DKA) with Kussmal respiration indicating acidosis, acetone on breath, with obtundation of consciousness or coma [16,17]. The revised diagnostic criteria for DM includes symptoms of DM plus random blood sugar > 200mg/dl (11.1mmol/L) or fasting plasma glucose > 126mg/dl (7.0mmol/) or 2-hour plasma glucose during oral glucose tolerance test > 200mg/dL [1,17].
T1DM is associated with long term damage, dysfunction or failure to various organs especially the eyes, kidneys, nerves, heart or blood vessels [1,3,4]. The acute complications are DKA and hypoglycemic reactions [3,16]. The chronic complications include retinopathy in 45 – 60% of >20 years disease and 20% after 10 years, nephropathy in 40% after 25 years disease and 50% of death in long term T1DM, with dwarfism and syndrome of limited joint mobility [17,18]. Long term management is a major challenge for child, family and health care team including education, insulin dosage, diet (nutritional requirement), exercise, stress management and blood glucose and urine ketone monitoring [1,2].
In Yemen there are no studies on childhood DM and the main objectives of this report is to highlight some baseline data on the clinical pattern, management, associated complications and outcome of TIDM among hospitalized children in Aden, Yemen.
Patients and Methods
All admitted children holding the diagnosis of T1DM with or without DKA were included in this study, during January 1999 through December 2001 at Al-Sadaka Teaching Hospital, Aden, Yemen. The patients’ demographic data, past history, clinical manifestations, investigations, management and outcome were obtained. Informed verbal consent was taken and ethical considerations was done by the hospital administration office. Data were entered into computer database SPSS Version 16 for windows. Statistical analysis included quantitative descriptive analysis as (Chi squares, Fischer exact test) and summary statistics (mean, percentages, and standard deviations).
Results
Overall 55 patients were holding the diagnosis of T1DM comprising a prevalence rate of 0.6% out of a total 9166 admitted patients during the study period. The male to female ratio was 1.5:1. The mean age (SD) was 10.5 (3.2). Sixty percent of the patients were admitted during the cool winter months. A higher proportion of the patients (47%) were from Aden governorate.
There was a documented consanguineous marriage with a positive family history of DM in 9 (16.4%) TIDM patients (Table 1). Five out of nine patients (55.6%) were of first degree relative and 4/9 children (44.4%) of second degree relative. There was a positive family history from male gender in 7/9 (77.7%), from father’s side in three patients (33.3%), one fraternal sibling (11%), two from grandfathers’ part (22.2%), one from grandmother’ side, one uncle and one aunt, each (11%) respectively. A higher proportion of patients 34 (61.8%) were diagnosed at peak age of 10-14 years with the youngest male infant of 16 months. Males with T1DM predominated in the three age groups of 0 – 4 years and 5 – 9 years and > 14 years while females outnumbered males in 10 – 14 years of age (Table 2). A significant difference of T1DM was observed between the different age groups and sex (p=0.04).
Country
Population /1000
Type 1 Diabetes Mellitus
< 15 years
>15 years
Egypt
64, 645
5.8
15.8
Libya
5784
1.0
2.6
Sudan
27518
1.1
1.1
Kuwait
1731
0.6
7.9
UAE
2308
0.1
1.1
Yemen
16294
0.4
0.3
Review article: NagatiK. Type 1 diabetes in IDF EMME Region Diabetes International 2000; 10 (2): 42-43 (14).
Table 1: Incidence of Type1 Diabetes Mellitus in different Arab Countries.
Demographic factors
N = 55 (100%)
Sex
Male
33 (60%)
Female
22 (40%)
Age (years)
Mean (SD)
10.5 (3.2)
Season
Winter
33 (60%)
Summer
22 (40%)
By years
1999
2000
2001
13 (24%)
23 (42%)
19 (34%)
Address
Aden
26 (47%)’
Lahej
15 (27%)
Abyan
11 (20%)
Shabwa
Mahra
1 (2%)
1 (2%)
Taiz
1(2%)
Family history
Positive
First degree relatives
Second degree relatives
9 (16.4%)
4 (7.3%)
5 (9.1%)
Table 2: Demographic characteristic of Yemeni children with Type 1 Diabetes Mellitus.
Under nutrition was evident in 44 (80%) of patients with 34 (62%) having body weights < 5th. percentile and 10 (18%) with weights <5th – 10th percentile. Overweight was recorded in 3 (5.5%) of the diabetic children (Figure 1). The presenting clinical manifestations were polydypsia and polyuria in 40 (73%) and 39 (71%) respectively followed by abdominal pain and weight loss in 25% of them. Polyphagia was referred in 13 (24%) and disturbance of consciousness in 9 (16.5%) of the patients (Table 3).
Age group in years
Sex
Male N (%)
Female N (%)
Total N (%)
0 – 4 yrs
3 (5.5%)
-
3 (5.5%)
5 – 9 yrs
12 (21.8%)
3 (5.5%)
15 (27.2%)
10 – 14 yrs
16 (29.1%)
18 (32.7%)
34 (61.8%)
>14yrs
2 (3.6%)
1 (1.8%)
3 (5.5%)
Total
33 (60%)
22 (40%)
55 (100%)
p=0.04
Table 3: Type 1 Diabetes Mellitus in Yemeni children by age and Sex.
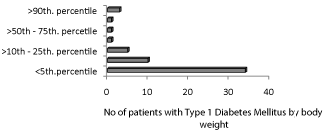
Figure 1: Type 1 Diabetes Mellitus in Yemeni children by body weight.
Random blood sugar was within the ketoacidosis range > 300mg/ dl in 32 (57.5%) and between 200 – 300mg/dl in19 (34.5%) of the patients (Table 4). None of the diabetic patients presented nor developed hypoglycemia during their hospital stay. All patients received insulin therapy. Short and intermediate acting insulin was prescribed for 43 (78%) patients, 11 (20%) received mixed insulin and one patient mixed insulin with short acting insulin.
Clinical presentation
Total N (%)
Polydypsia
40 (73%)
Polyuria
39 (71%)
Abdominal pain
15 (25.7%)
Weight loss
14 (25.5%)
Polyphagia
13 (24%)
General weakness
13 (24%)
Disturbed consciousness
9 (16.5%)
Fever
8 (14.5%)
Breathlessness
8 (14.5%)
Vomiting
7 (13%)
Vertigo
7 (13%)
Table 4: Type1 Diabetes Mellitus among Yemeni children by clinical Presentation.
Sixteen patients developed adverse complications and diabetic ketoacidosis comprised the higher percentage in 11 (20%) of the patients. 48 (87.3%) were discharged in good condition but 2 (3.6%) died due to DKA (Table 5). The mean hospital stay in children with T1DM was 13.6 (7.3) days with a higher proportion 19 (34.5%) for 1 to 2 weeks (Table 6).
Blood sugar mg/dL
Patients N (%)
<70
-
70 – 200
4 (7%)
>200 – 300
19 (34.5%)
>300 – 500
31 (56%)
>500
1 (1.5%)
Total
55 (100%)
Table 5: Type 1 Diabetes Mellitus in Yemeni children by random blood sugar results.
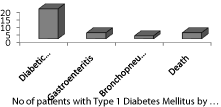
Figure 2: Type 1 Diabetes Mellitus in Yemeni children by Complications.
Hospital stay
Patients N (%)
0 – 7 days
16 (29%)
8 – 14 days
19 (34.5%)
15 – 22 days
12 (22%)
23 – 30 days
7 (13%)
>30 days
1 (1.5)
Total
55 (100%)
Table 6: Type 1 Diabetes Mellitus in Yemeni children by hospital stay.
Discussion
T1DM is a chronic disease that requires long-term medical attention both to prevent the development of its overwhelming complications and to manage them when they do occur. The prevalence rate of T1DM in this hospital based study was 0.6% among Yemeni patients but it does not reflect the actual conditions in the community; however it remains comparably higher to reports in Algeria (0.03%) and India (0.03%) [19,20]. The male to female ratio was 1.5:1 in this study consistent with the reports from Algeria (1.6:1), Sudan (1.5:1) and India (1.5:1) [19,21,22,23]. Females outnumbered males in Pakistan, Kuwait and Oman [16,24,25], but there was no sex difference in UAE and Finland [5,19]. This gender difference might be related to difference in geographic distributions or probably may be overestimated since males are overprotected and receive great attention than females in our society.
The recognized seasonality of T1DM is the winter peak in this study consistent with the literature reports [3,26]. Multiple environmental and genetic factors play an important role in the development of the disease [5,7,12,27]. A significant studied patients were from Aden governorate (47%) where the hospital is centrally located, followed by Lahej (27%) with scattered patients from neighbouring governorates, since the inhabitants of different districtsand governorates are dependent on the central referralpediatric teaching hospital.
A positive family history of 16.4% in T1DM patients in this study was higher than that in South India (9.9%) but lower than Kuwait (31%), and Oman (28%) [20,24,25]. It may be due to lack of available data from the families who could not recall or did not know their positive family history.
Several authors have documented the gender role in association with the disease transmission [28,29]. It is reported that the risk from the father is almost two to three times higher than that of the diabetic mother [3,12,28,29]. In the present study the role of male gender is supported by the evidence of 7 out of 9 (77.7% ) of the patients acknowledging evidence of positive consanguineous marriage together with a positive family history of DM from males both from the first and second degree relatives.
There are two peak ages of T1DM at 5 – 7 years and puberty which coincide with the present study revealing 27.3% and 61.8% respectively [7,12]. This peak prevalence is attributed to the increased pubertal hormones and emotional stress occurring at this age [3,5,23]. These variations may be explained by the effect of several environmental factors associated with the disease onset. Many authors have observed no correlation between the socioeconomic status and T1DM suggesting the facts that poor socioeconomic class inhabitants are usually protected against DM [21,30,31]. However, this explanation does not agree with this study where under nutrition was significantly evident in 80% of the patients while overweight was recorded in only 5.5% of the diabetic children. A higher proportion of our patients are from poor socioeconomic status. Hence future studies are required to further investigate whether the role of socioeconomic status has any correlated effect on T1DM.
In this study the classical manifestations of T1DM were polydypsia (73%) and polyuria (71%), followed by abdominal pain (25%), weight loss (25%) and polyphagia (24%). Ketoacidosis was a major complication in (20%) where (16.5%) developed disturbance of consciousness. These typical manifestations did not differ from the classical findings in T1DM in the literature [6,16,21]. Insulin therapy is the cornerstone management of T1DM and all TIDM patients were treated with human insulin. A higher proportion of patients 78% were treated with short and intermediate acting insulin, (20%) received mixed insulin and only one patient received mixed insulin with short acting insulin.
Despite major advances in the care of T1DM in developed countries, DKA remains a leading cause of hospitalization among children with T1DM [3,12,16]. In the present study 87% of TIDM children were discharged in a stabilized condition. The mean hospital stay was 13.6 days with a prolonged hospital stay more than three weeks in all patients with DKA that followed similar trends in many countries [17,32]. DKA was the major adverse complication in 11 (20%) resulting in 3.6% of death higher than that in industrialized countries but within the range of developing countries [3,16,26,33]. This high mortality may be explained by the fact that the differences in the severity of the clinical findings might also appear because of low disease awareness in countries with low prevalence that might contribute to a delayed diagnosis. Moreover, these mortality variations may be related to several factors including health care delivery systems and socioeconomic status in these countries [32, 33]. Thus, TIDM is a current stressful issue for Yemeni children between 10 – 14 years of age. A high risk of DM in affected families supports the role of genetic factors. DKA was one major complication and a leading cause of hospitalization that accounted for 3.6% of death cases.
Type1DM is a chronic disease that requires patience and time from health professionals and families for its management. This is the first report on T1DM among children in Yemen and these baseline results might help to determine public health polices in the country to improve the diagnosis of T1DM and provide prompt diagnostic and therapeutic strategies to make better patient’s quality of life and their outcome.
Conclusion and Recommendations
It is noteworthy to establish a diabetic registry in Aden. Provision of best health services available and affordable to every diabetic child is highly recommended. Intensified health education about early symptoms of DM in the general population and among general practitioners is needed to prevent serious metabolic decomposition at onset. Further future research studies are required to assess the magnitude of the problem of T1DM among children as it becoming an important health burden in this community.
Acknowledgment
Authors would like to thank children, stuff nurse and archives workers in Al-Sadakateaching hospital of Aden governorate, Yemen for their cooperation in this study.
References
- Alemzadeh R, Wyatt DT. Diabetes mellitus in: Behrman RE, Kleigman RM, Jenson HB, eds. Nelson Textbook of Paediatrics. 17th. Ed. Philadelphia, WB Saunders 2004.
- Reinauer H, Home PD, Kanangasabapathy AS, Heuck CC. Laboratory diagnosis and monitoring of diabetes mellitus 2002 in : World health organization Geneva, Laboratory diagnosis and monitoring of diabetes mellitus. 2002; 5-25.
- Abdul-Rasoula M, Al-Mahdib M, Al-Qattanb H, Al-Tarkait N, Alkhouly M, Al- Safi R, et al. Ketoacidosis at presentation of Type 1 diabetes in children in Kuwait: frequency and clinical characteristics. Paediatric Diabetes. 2010; 11: 351-356.
- Warehan NJ, O’Rahily S. The changing classification and diagnosis of diabetes new classification is based on pathogenesis not insulin dependence. BMJ. 1998; 317: 359-360.
- Punnose J, Agarwalb MM, ElKhadirc A, Devadasc K, Mugamera IT. Childhood and adolescent diabetes mellitus in Arab residing in the United Arab Emirates. Diabetes research and clinical practice. 2002; 55: 29 -33.
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice. 2010; 87: 4-14.
- Karvonen M, Viik-Kajander M, Moltchnova E, Libman I, Laporte R, Tuomilehto J. For the Diabetes Mondiale (DIAMOND) project group. Incidence of Childhood Type 1 Diabetes Worldwide. Diabetes Care. 2000; 23: 1516-1526.
- Sadauskaite V,_Kuehne, Samuelsson U, Padaiga Z, Urbonaite B, Edenvall H, et al. Severity at onset of childhood type 1 diabetes in countries with high and low incidence of the condition. Diabetes Research and Clinical Practice. 2002; 55: 247-254.
- Tobon GJ, Arango A, Abad V. Clinical and immunological characteristics of type 1 diabetes mellitus in a northwestern Colombian population. Diabetes Research and Clinical Practice. 2006; 72: 170-175.
- Schrezmenmeir J, Jagla A. Milk and diabetes. Journal of the American college of Nutrition. 2000; 19: 176-190.
- Gerstein HC. Cow’s milk exposure and type 1 diabetes mellitus, a critical review of the clinical literature. Diabetes care. 1994; 17: 13 -19.
- Lone SW, Siddiqui EU, Muhammed F, Atta I, Ibrahim MN, Raza J. Frequency, clinical characteristics and outcome of diabetic ketoacidosis in children with type-1 diabetes at a tertiary care hospital. J Pak Med Assoc. 2010; 60: 725- 729.
- Bui TP, Werther GA, Cameron FJ. Trends in diabetic ketoacidosis in childhood and adolescence: a 15-year experience. Paediatric Diabetes. 2002; 3: 82-88.
- Menson PSN, Ghai OP, Gupta P, Paul VK, eds. Diabetes Mellitus. Ghai Essential Paediatrics. 5th. ed. Delhi, Metha publishers. 2001; 434-440.
- Francis J, Rose SJ, Raafat F, Milford DV. Early onset of diabetic nephropathy. Arch Dis Child. 1997; 77: 524- 525.
- Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Diabetes Mondiale (DiaMond) Project Group, Incidence of childhood Type1diabetes worldwide. Diabetes Care. 2000; 23:1526-1526.
- Asha Bai PV, Krishnaswami CV, Chellamariappan M, Kumar GV, Subramaniam JR. Glycosuria and diabetes mellitus in children and adolescents in south India. Diabetes Research and Clinical Practice. 1991; 13: 131-136.
- Elrayah H, Eltom M, Bedri A, Belal A, Rosling H, Ostenson CG. Economic burden on families of childhood type 1 diabetes in urban Sudan. Diabetes Research and Clinical Practice. 2005; 70: 159-165.
- Elamin A, Altahir H, Ismail B, Tuvemo T. Clinical pattern of childhood Type 1 (insulin-dependent) diabetes mellitus in the Sudan. Diabetologia. 1992; 34:645-648.
- Kalra S, Kalra B, Sharma A. Prevalence of type 1 diabetes mellitus in Karnal district, Haryana state, India. Diabetology& Metabolic Syndrome. 2010; 2: 14-16.
- Abdul-Rasoul M, Al-Qattan H, Al-Haj A, Habib H, Ismael A. Incidence and seasonal variation of Type 1 diabetes in children in Farwania area, Kuwait (1995–1999). Diabetes Research and Clinical Practice. 2002; 56: 153-157.
- Shaikh H, Bappal B, Nair R, Al Khusaiby S. A Five Year Study of the Incidence and Familial Characteristics of Insulin Dependent Diabetes Mellitus in the Muscat Region of Sultanate of Oman. The 14th International Child Health Conference of Recent Advances in Child Health. Muscat, Oman. 2001; February: 5-7.
- Glaser NS, Wootton-Gorges SL, Buonocore MH, Marcin JP, Rewers A, Strain J, et al. Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Paediat Diabetes. 2006; 7: 75-80.
- Davis TME, Bruce DG, Davis WA. Prevalence and prognostic implications of the metabolic syndrome in community-based patients with type 1 diabetes: Diabetes Research and Clinical Practice. 2007; 78: 412-417.
- Chuang LE, Tsai ST, Juang JH, Tsai WY, Tai TY. Genetic epidemiology of type 1 diabetes mellitus in Taiwan. Diabetes Research and Clinical Practice. 2000; 50: S41-S47.
- Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, et al. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009; 41: 703-707.
- Walsh MG, Zgibora J, Songer T, Borch-Johnsen K, Orchard TJ. The socioeconomic correlates of global complication prevalence in type 1 diabetes (T1D): A multinational comparison. Diabetes research and clinical practice. 2005; 70:143-150.
- Soria J, Garagorri JM, Rodriguez M, Rodriguez G, Larrad L, Elizalde M. Epidemiology and genetic risk of type 1 diabetes among children in Aragon community, Spain. Diabetes research and clinical practice. 2008; 79: 112- 116.
- Wolfsdorf J, Craig M, Daneman D et al. ISPAD Clinical practice consensus guidelines 2006-2007. Diabetes ketoacidosis. Pediatr Diabetes. 2007; 8: 28- 43.
- Curtis JR, Muirhead TS, Cummings E, Daneman D. Recent trends in hospitalization for diabetic ketoacidosis in Ontario children. Diabetes Care. 2002; 25:1591-1596.