
Research Article
J Pediatri Endocrinol. 2017; 2(2): 1019.
Characteristics and Ethnic Variations of Adolescents with Polycystic Ovarian Syndrome at a Tertiary Care Center
Shenep L* and Al-Zubeidi H
Department of Pediatrics, University of Tennessee Health Science Center, USA
*Corresponding author: Shenep L, Department of Pediatrics, Memphis, University of Tennessee Health Science Center, TN, USA
Received: July 09, 2017; Accepted: November 24, 2017; Published: December 01, 2017
Abstract
Objective: Determine the prevalence, characteristics, and ethnic variations in adolescents with Polycystic Ovarian Syndrome (PCOS).
Methods: A retrospective chart review of 250 adolescent girls with a diagnosis of PCOS or related co-morbidities between April 2014 to April 2016 was performed. A total of 92 patients were identified as having either possible PCOS or confirmed PCOS.
Results: The prevalence of confirmed PCOS in our cohort was 0.22%, and the prevalence of confirmed and possible PCOS was 1%. The average age was 15.4 years, and 90% of patients had a BMI>85%. 65% were black and 26% white. Black subjects had a greater prevalence of more severe obesity, defined as a BMI>40 (p=0.014), and a higher prevalence of elevated hemoglobin A1c (p=0.003). White subjects had a higher prevalence of an abnormal lipid profile (p=0.004). In black patients, hemoglobin A1C was positively associated with free testosterone (p=0.016), and BMI z score was negatively associated with sex hormone binding globulin (p=0.001).
Conclusion: Ethnicity influences the metabolic phenotype of PCOS within adolescents. Metabolic characteristics such as BMI and hemoglobin A1c are correlated with hyperandrogenemia.
Keywords: Polycystic ovarian syndrome; Ethnic variation; Adolescents
Introduction
Polycystic Ovarian Syndrome (PCOS) is one of the most common metabolic disorders in women of reproductive age. It is characterized by ovarian hyperandrogenism and chronic anovulation. While the pathogenesis of PCOS is not fully understood, PCOS likely develops from a combination of genetic and environmental factors affecting steroid metabolism [1]. Abnormalities in ovarian steroidogenesis as well as insulin resistance play a role in the development of hyperandrogenism [2-4].
Most studies regarding ethnic variations in patients with PCOS are limited to adult populations [5]. Ethnic variations in insulin resistance, obesity, lipid profiles have been described in women with PCOS [5-10]. In general, higher rates of obesity and insulin resistance are observed in black women with and without PCOS [7,8,10- 12]. Few studies have shown ethnic differences in androgens and gonadotropin [5].
Many features of PCOS overlap with the characteristics of normal pubertal development, including irregular menstrual cycles, acne, and polycystic ovarian morphology, making the diagnosis challenging in this age group [2]. Three international conferences have developed diagnostic criteria for adult women, including the National Institutes of Health (NIH) conference criteria (1990), the Rotterdam consensus criteria (2003), and the Androgen Excess-PCOS Society consensus criteria (2006) [13-15]. In 2003, an Endocrine Society-appointed Task Force suggested that the diagnosis of PCOS in adolescents be made based on the presence of clinical and/or biochemical Hyper- Androgenism, persistent oligomenorrhea, and exclusion of related disorders, which is consistent with the NIH criteria [16].
The objective of this study is to estimate the prevalence of PCOS and baseline characteristics within an adolescent population at a tertiary care center in Memphis, TN. We also aim to evaluate ethnic variations on clinical, hormonal, and metabolic parameters.
Methods
Study design
This was a retrospective chart review, conducted in an academic health center setting, designed to evaluate the prevalence, characteristics, and ethnic variations in adolescents with Polycystic Ovarian Syndrome (PCOS). The institutional review board of Le Bonheur Children’s hospital, Memphis, TN approved this study.
Subjects
A total of 9,205 girls aged 12-19 between April 2014 and April 2016 were seen in the General Pediatrics Clinic, Healthy Lifestyles Clinic, and Pediatric Endocrinology Clinic at Le Bonheur Children’s Hospital, Memphis, TN. We identified 250 subjects who received a diagnosis of PCOS [International Classification of Diseases, Ninth Revision (ICD-9) 256.4 or Tenth Revision (ICD-10) E28.2] or a related disorder, including amenorrhea (626.0 or N91.5), scanty or infrequent menstruation (626.1), irregular menstrual cycle (626.4 or N92.6), hyperandrogenism (256.1), or hirsutism (704.1 or L68.0).
Of the 250 subjects identified by ICD-9 or ICD-10 codes, a total of 92 subjects were included in the study. Those subjects were divided into two groups: confirmed-PCOS and possible- PCOS. The confirmed-PCOS group consisted of 20 adolescents who had biochemical hyperandrogenism, menstrual dysfunction (oligomenorrhea or menorrhagia), and exclusion of related disorders (which required a normal 17-hydroxyprogesterone level). The possible-PCOS group consisted of 72 adolescents who met at least one criterion but may have had an incomplete evaluation. Of those, 24 subjects had biochemical Hyper- Androgenism only, 28 subjects had menstrual dysfunction only, and 20 subjects had menstrual dysfunction and biochemical Hyper-Androgenism but without exclusion of related disorders. The confirmed-PCOS and the possible- PCOS groups were combined for the analysis and named all-PCOS. Characteristics of the study population are shown in (Table 1). The remainder (158 subjects) were excluded. Exclusion criteria included: 1) normal androgen laboratory studies or had another known cause of abnormal androgens, including congenital adrenal hyperplasia; 2) pregnancy or a history of pregnancy; or 3) menstrual irregularity secondary to abnormal thyroid studies, poorly controlled type 1 diabetes, prolactinoma, or the use of certain medications, including Depo-Provera and anti-psychotics. Subjects were not excluded if they were taking oral contraceptive pills or metformin.
Variables
Mean +/- SD
Normal Range
Age (yrs)
15 +/-2
Menarche (yrs)
11 +/-1
BMI z score
2 +/- 0.7
Total Testosterone (ng/dL)
67 +/-40
20 – 38
Free Testosterone (pg/mL)
11 +/- 9
1.1 – 6.3
SHBG (nmol/L)
28 +/- 17
36 – 125
LH (mIU/mL)
9.7 +/- 8
0 – 11.7
Triglycerides (mg/dL)
113 +/-64
50 – 209
Total Cholesterol (mg/dL)
161 +/-28
124 – 212
HDL (mg/dL)
42 +/-11
27 – 70
LDL (mg/dL)
93+/-26
61 – 131
Hemoglobin A1c (%)
5.41 +/-0.44
4 – 6
Table 1: Characteristics of subjects with all-P.
Outcome measures
Information was extracted from each chart, including age, age of menarche, race, Body Mass Index (BMI), medications, and laboratory studies. An elevated hemoglobin A1c was defined as greater than or equal to 5.7%. An abnormal lipid profile was defined as one of the following: triglycerides greater than 209 mg/dL, total cholesterol greater than 212 ng/dL, HDL less than 27 mg/dL, or LDL greater than 131 mg/dL. The majority of patients (approximately 80%) had laboratory data using the same assay, while the remaining patients had laboratory studies obtained from outside sites. Total testosterone, free testosterone, Sex Hormone Binding Protein (SHBP), and 17a-hydroxyprogesterone were primarily measured via High Performance Liquid Chromatography/Tandem Mass Spectrometry (HPLC/MS-MS), equilibrium dialysis at Esoterix, Incorporated (Calabasas Hills, CA).
Statistical methods
Differences in the distribution of demographics, laboratory parameters, and presence of co-morbidities between groups were assessed with chi-squared tests for categorical variables and non-parametric independent samples Mann-Whitney U tests for continuous variables. We assessed the effect of BMI and of hemoglobin A1C by performing linear regressions. All analyses were conducted using the Statistical Package for The Social Sciences (SPSS) for Windows, Version 23 (IBM Corp, Armonk, NY). P values <0.05 were considered significant.
Results
The prevalence of confirmed-PCOS was 0.22%, and the prevalence of all-PCOS was 1%. The all-PCOS group was used for the analysis. The average age was 15.4 years. Other distinguishing characteristics included: 65% were black, 26% were white, and 9% were “other” race; 3% were overweight and 87% were obese; 39% had a BMI>40 mg/ m2; 26% subjects (11 subjects) had a hemoglobin A1c greater than or equal to 5.7-6.5%. Two subjects had a diagnosis of Type 2 diabetes. Additional characteristics are shown in (Table 1).
Of the patients who met criteria for confirmed PCOS, only 40% received a diagnostic code for PCOS. There were no significant differences in the age, androgen level, BMI z score, hemoglobin A1c, or lipid profile between subjects who received a diagnostic code for PCOS compared those who did not receive a diagnostic code for PCOS.
Black subjects had a higher prevalence of severe obesity defined as a BMI>40kg/m2 compared to white subjects (50% vs. 21%, p=0.014). Only black subjects had a hemoglobin A1C greater than or equal to 5.7%. An abnormal lipid profile was more common in white subjects compared to black subjects (56% vs. 11%, p=0.04). White subjects had a higher mean triglyceride level (180 vs. 92 mg/dL, p<0.001) and lower mean HDL (36 vs. 43 mg/dL, p =0.04) compared to black subjects. Black compared to white subjects had no significant difference in the over-all prevalence of obesity (95% vs. 79%, p=0.11) except for severe obesity rates. There were no ethnic differences between black and white subjects with all-PCOS for demographic variables or androgen levels (Table 2).
Characteristic
Black
White
PCOS diagnosis
13/60 (22%)
7/24 (30%)
BMI>85%
57/60 (95%)
19/24 (79%)
BMI > 40
30/60 (50%)*
5/24 (21%)
HgbA1C >/= 6.5%
2/42 (5%)*
0/19 (0%)
HgbA1C >/= 5.7%
11/42 (26%)*
0/19 (0%)
Abnormal lipid profile
4/38 (11%)*
5/9 (56%)
*P<0.05 compared with white race.
Table 2: Ethnic variations in subjects with all-PCOS.
Linear regressions were performed to determine the relationship between metabolic variables (including BMI and hemoglobin A1c) and androgens. Significant associations were only observed for black subjects. There was no significant direct association between BMI z scores with androgen levels, but there was a negative association between BMI z score and SHBP. The slope of the BMI z score on SHBP was negative: R=-0.63 (p=0.0001) (Figure 1). The slope of the hemoglobin A1C on SHBP was also negative: R=-0.36 (p=0.048) (Figure 2), and, the slope of the hemoglobin A1c on free testosterone was positive: R=0.46 (p=0.016) (Figure 3 and Table 3).
Characteristic
Black
White
Total Testosterone (ng/dL)
66.56 +/- 36.20
68.54 +/- 53.02
Free Testosterone (pg/mL)
10.57 +/- 8.28
13.19 +/- 11.50
Sex Hormone Binding Globulin (nmol/L)
29.44 +/- 19.53
27.07 +/- 11.79
Triglycerides (mg/dL)
92.35 +/- 46.51*
180.22 +/- 66.90
Total Cholesterol (mg/dL)
158.87 +/- 28.13
167.67 +/- 28.15
HDL (mg/dL)
43.03 +/- 11.17*
36.11 +/- 6.72
LDL (mg/dL)
90.08 +/- 25.55
103.44 +/- 27.3
HgbA1C (%)
5.45 +/- 0.45
5.22 +/- 0.26
Data are presented as the mean +/- standard deviation.
*P<0.05 compared with white race.
Table 3: Laboratory parameters by ethnicity in subjects with all-PCOS.
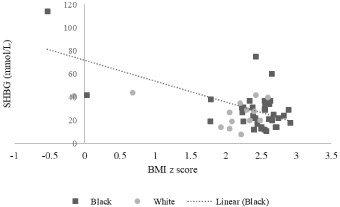
Figure 1: Correlation between BMI z score and SHBG. The slope of BMI z
score on SHBP was negative, and significant for black subjects: R=-0.633
(p=0.00) but not significant for white subjects (p=0.168).
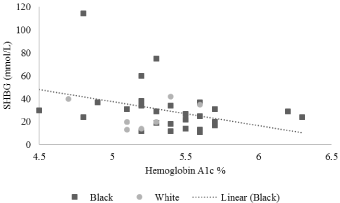
Figure 2: Correlation between Hemoglobin A1c and SHBG. The slope of the
hemoglobin A1c on SHBG was negative, and significant for black subjects:
R=-0.364 (p=0.048) but not significant for white subjects (p=0.911).
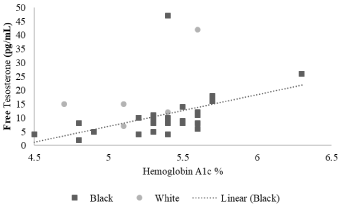
Figure 3: Correlation between hemoglobin A1c and free testosterone. The
slope of the hemoglobin A1c on free testosterone was positive, and significant
for black subjects: R=0.458 (p= 0.016) but not significant for white subjects
(p=0.281).
Discussion
The estimated prevalence of PCOS in adolescents in our center approximates prevalence estimates of larger scale studies. Christensen, et al. reported a prevalence of 0.56-1.14 in a cross-sectional study of 137,502 subjects 15-19 years old in southern California [17]. Lo, et al. reported a prevalence of 0.81% in subjects 15-19 years old in a population of 104,729 subjects who were a part of an integrated health care delivery system in northern California [10]. While, in this study, there were no differences between subjects who received a diagnostic code for PCOS from those who did not, one large scale study did find that obese adolescent girls were more likely to receive a diagnostic code for PCOS compared to adolescent girls with a normal weight [17]. The authors of this study discussed the potential for bias that may result in an overestimation of the association between PCOS and obesity [17]. In this study, only 40% of patients with confirmed PCOS received a diagnostic code for PCOS. This discrepancy may in part be due to the inability of the provider to specify the diagnosis at the clinic visit until all laboratory data was reviewed. This finding is important because it indicates that a search of subjects using only the diagnostic code for polycystic ovarian syndrome and without related diagnoses, such as hirsutism and oligomenorrhea, would underestimate the population for review.
The criteria used in this study to define confirmed-PCOS are more stringent than criteria used in other studies. This study required the presence of biochemical Hyper-Androgenism, while other studies accept clinical Hyper-Androgenism [5,7,9,10,17,18]. However, because this study is retrospective, it is difficult to standardize the criteria for clinical Hyper-Androgenism. Ferriman-Galloway scoring for hirsutism is rarely documented in subject charts, and has a range of normal variations depending on ethnicity [19]. For confirmed- PCOS, we also required a normal value for 17-hydroxyprogesterone to rule out non-classical CAH. More than one third of the possible- PCOS group was excluded from the confirmed-PCOS group due to lacking a value of 17-hydroxyprogesterone level.
In this study, we report ethnic variations in the metabolic profile of adolescents with PCOS. We noted a higher prevalence of severe obesity, elevated hemoglobin A1c, and a more favorable lipid profile in black subjects compared to white subjects. Studies of women both with and without PCOS have demonstrated similar ethnic variations in metabolic features. For example, multiple studies have found a higher average BMI in black women compared to white women with PCOS [6,8,10]. Black adolescent females in general have also been observed to have a higher BMI and a greater incidence of type 2 diabetes compared to white adolescent females [11,12]. Welt et al. found that African American women with PCOS were most likely to have a diagnosis of type 2 diabetes mellitus and a higher insulin level compared to other ethnic groups, although they did not demonstrate a difference with impaired glucose tolerance [5]. Kovel, et al. found that black women with PCOS have a more favorable lipid profile than white women with PCOS [18]. Other studies have demonstrated a more favorable lipid profile in black subjects compared to white subjects regardless of having PCOS [20,21]. Thus, the main metabolic differences that we found between black and white subjects in our study are consistent both with patterns observed in the ethnic populations in general and in studies of women with PCOS. While not fully understood, the ethnic differences in insulin resistance and dyslipidemia between black and white subjects may be related to a combination of factors, including genetic and environmental variables.
In this study, linear regressions demonstrated significant correlations between metabolic variables and androgen levels only in black subjects, likely due to the greater number of black subjects in our study. Other studies have demonstrated similar associations between metabolic factors and androgens. Welt et al. found that in adult women, insulin correlated directly with testosterone and indirectly with SHBP within their Icelandic subjects but not their Boston subjects [5]. The correlation we observed between androgens and metabolic variables of BMI and hemoglobin A1c are consistent with the proposed pathophysiology of hyperinsulinemia promoting Hyper-Androgenism in some subjects with PCOS.
This study was a retrospective review of a small cohort of patients and was therefore limited by variability in available laboratory data and incomplete documentation of menstrual histories. However, we believe that it provides new insight into the characteristics of PCOS within the adolescent population, such as demographics, comorbidities, and diagnostic coding. Our findings also contribute to the discussion regarding the association between ethnicity and metabolic variables on androgens and other reproductive hormones. Further studies are needed to characterize ethnic variations in these metabolic and reproductive factors contributing to the development of PCOS, especially within the adolescent population.
References
- Rosenfield RL. The Diagnosis of Polycystic Ovary Syndrome in Adolescents. Pediatrics. 2015; 136: 1154-1165.
- Witchel SF, Oberfield S, Rosenfield RL, Codner E, Bonny A, Ibanez L, et al. The Diagnosis of Polycystic Ovary Syndrome during Adolescence. Horm Res Paediatr. 2015.
- Nelson VL, Legro RS, Strauss JF 3rd, McAllister JM. Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol. 1999; 13: 946-957.
- Ibanez L, Ong KK, Lopez-Bermejo A, Dunger DB, de Zegher F. Hyperinsulinaemic androgen excess in adolescent girls. Nat Rev Endocrinol. 2014; 10: 499-508.
- Welt CK, Arason G, Gudmundsson JA, Adams J, Palsdottir H, Gudlaugsdottir G, et al. Defining constant versus variable phenotypic features of women with polycystic ovary syndrome using different ethnic groups and populations. The Journal of clinical endocrinology and metabolism. 2006; 91: 4361-4368.
- Carmina E, Koyama T, Chang L, Stanczyk FZ, Lobo RA. Does ethnicity influence the prevalence of adrenal hyperandrogenism and insulin resistance in polycystic ovary syndrome? Am J Obstet Gynecol. 1992; 167: 1807-1812.
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. The Journal of clinical endocrinology and metabolism. 1998; 83: 3078-3082.
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. The Journal of clinical endocrinology and metabolism. 2004; 89: 2745-2749.
- Essah PA, Nestler JE, Carmina E. Differences in dyslipidemia between American and Italian women with polycystic ovary syndrome. J Endocrinol Invest. 2008; 31: 35-41.
- Lo JC, Feigenbaum SL, Yang J, Pressman AR, Selby JV, Go AS. Epidemiology and adverse cardiovascular risk profile of diagnosed polycystic ovary syndrome. The Journal of clinical endocrinology and metabolism. 2006; 91: 1357-1363.
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012; 307: 483-490.
- Writing Group for the SfDiYSG, Dabelea D, Bell RA, D’Agostino RB Jr, Imperatore G, Johansen JM, et al. Incidence of diabetes in youth in the United States. JAMA. 2007; 297: 2716-2724.
- Zawadzki J DA. Diagnostic Criteria for polycystic ovarian syndrome: towards a rational approach. Boston. 1992.
- Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004; 81: 19-25.
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009; 91: 456-488.
- Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2013; 98: 4565-4592.
- Christensen SB, Black MH, Smith N, Martinez MM, Jacobsen SJ, Porter AH, et al. Prevalence of polycystic ovary syndrome in adolescents. Fertil Steril. 2013; 100: 470-477.
- Koval KW, Setji TL, Reyes E, Brown AJ. Higher high-density lipoprotein cholesterol in African-American women with polycystic ovary syndrome compared with Caucasian counterparts. The Journal of clinical endocrinology and metabolism. 2010; 95: 49-53.
- Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981; 140: 815-830.
- Santos RD, Bensenor IM, Pereira AC, Lotufo PA. Dyslipidemia according to gender and race: The Brazilian Longitudinal Study of Adult Health (ELSABrasil). J Clin Lipidol. 2016; 10: 1362-1368.
- Bauman WA, Adkins RH, Spungen AM, Maloney P, Gambino R, Waters RL. Ethnicity effect on the serum lipid profile in persons with spinal cord injury. Arch Phys Med Rehabil. 1998; 79: 176-180.