
Research Article
J Pediatri Endocrinol. 2017; 2(2): 1020.
Trends in the use of Puberty Blockers among Transgender Children in the United States
Lopez CMD*, Solomon S, Boulware RA, Cowles DE, Ozgediz DH, Stitelman MG, Caty ER and Christison L
Department of Surgery, Section of Pediatric Surgery, Yale University School of Medicine, USA
*Corresponding author: Lopez CMD, Department of Surgery, Section of Pediatric Surgery, Yale University School of Medicine, USA
Received: November 11, 2017; Accepted: December 22, 2017; Published: December 29, 2017
Abstract
Objective: To identify national trends in the utilization of histrelin acetate implants among transgender children in the United States
Methods: We analyzed demographic, diagnostic, and treatment data from 2004 to 2016 on the use of Histrelin Acetate reported to the Pediatric Health Information System (PHIS) to determine the temporal trends in its use for transgender-related billing diagnoses, e.g. “Gender Identity Disorder.”Demographic and payer status data on this patient population was also collected.
Results: Between 2004 and 2016, the annual number of implants placed for a transgender related diagnosis increased from 0 to 63. The average age for placement was 14 years. Compared to natal females, natal males were more likely to receive implants (57 versus 46) and more likely to have implants placed at an older age (62% of natal males versus 50% of natal females were>13 years; p<0.04).The majority of children were white non-Hispanic (white: 60, minority: 21). When compared to the distribution of patients treated for precocious puberty (white: 1428, minority: 1421), white non-Hispanic patients were more likely to be treated with a histrelin acetate implant for a transgender related diagnosis than minority patients (p<0.001). This disparity was present even among minority patients with commercial insurance (p<0.001).
Conclusion: Utilization of histrelin acetate implants among transgender children has increased dramatically. Compared to natal females, natal males are more likely to receive implants and also more likely to receive implants at an older age. Treated transgender patients are more likely to be white when compared to the larger cohort of patients being treated with histrelinacetate for Central Precocious Puberty (CPP), thus identifying a potential racial disparity in access to medically appropriate transgender care.
Keywords: Transgender children; Histrelin acetate; Disparities; Race; Payer status
Introduction
Long-acting gonadotropin releasing hormone analogues (GnRHa’s), often referred to as “puberty blockers”, to suppress endogenous sex hormone production are FDA-approved for the treatment of Central Precocious Puberty (CPP). In recent years, however, the use of these agents has expanded to include a growing number of transgender children. In Tran’s youth, GnRHa therapy suppresses normally timed pubertal development. In these individuals, the onset of pubertal progression and the resultant phenotypic acquisition of secondary sex characteristics has been shown to be associated with increased anxiety, depression, and gender dysphoria [1]. Moreover, many of these changes have limited reversibility, decreasing the subsequent effectiveness of cross hormone therapy.
The most commonly used medications for pubertal suppression are leuprolide acetate injections (Lupron; AbbVie, Chicago, IL) andhistrelin acetate subcutaneous implant (Supprelin, Vantas; Endo Pharmaceuticals, Malvern, PA). The safety and efficacy of these agents have been reported in a number of populations and both regimens are recommended in the World Professional Organization for Transgender Health Standards of Care and the Endocrine Society Guidelines [2-7].
While appropriate medical intervention along with an affirming environment has been shown to result in improved health outcomes for transgender individuals, national survey data on the number of transgender children remains elusive andtrends in the utilization of hormone suppressing therapies in this population have not previously been described [1-4].
We conducted a study to analyze the temporal trends in the number of histrelin acetate implants placed in children in the United States for a transgender-related diagnosis and to examine differences in its utilization among transfemale (natal male) and transmale (natal female) individuals.
Methods
Study population and design
The study was a retrospective data analysis using the Pediatric Health and Information System (PHIS) database, a comprehensive pediatric database operated by the Children’s Hospital Association. The database includes clinical, demographic, and financial details of more than six million patients in 43 US children’s hospitals, including data from major US centers with specialized pediatric gender clinics [8]. The diagnostic codes for Gender Identity Disorder (ICD-10 F64.00) and Sexual and Gender Identity Disorders/Gender Dysphoria (ICD-9 302.00) were used as a proxy for transgender status. The PHIS database was searched for patients who were admitted for placement of a histrelin acetate implant (CPT 11981) in association with a principle diagnosis of F64.00 and 302.00 from 2004 to 2016. Patients younger than 19 years of age were included in the study. The age at admission, utilization of histrelin acetate, age at implant placement, natal sex, and the hospital of admission were obtained. For comparison, PHIS data on patients admitted for placement of a histrelin acetate implant (CPT 11981) in association with a diagnosis of precocious puberty (ICD-10 E30.1; ICD-9 259.1) was also gathered. This study was submitted and approved by the hospital’s institutional review board (HIC# 2000021331).
Pilot validation of search criteria
Medical records of patients admitted to Yale New Haven Children’s Hospital from February 2014 to June 2017 for surgical placement of a histrelin acetate implant were identified. A total of 92 patient cases (n=2 Transyouth, n=81CPP, n=1 Pituitary Dwarfism, n=1 Short Stature, n=7 Other) were manually reviewed to identify transgender children and associated ICD diagnostic codes. ICD codes for Gender Identity Disorder (ICD-10 F64.00) and Sexual and Gender Identity Disorders/Gender Dysphoria (ICD-9 302.00) correctly identified transgender children.
Statistical analysis
A thorough analysis of demographics associated with histrelin acetate use in the pediatric transgender population was conducted. Continuous data were summarized with means and standard deviations. Proportions were compared using the fisher exact twotailed tests. The p value was set at 0.05.
Results
The population of children undergoing histrelin acetate implants for a transgender-related diagnosis included 92 unique patients from 12 hospitals. The population of children undergoing histrelin acetate implants for precocious puberty included 2240 unique patients from 34 hospitals.
Notable temporal differences were found with regard to the incidence of histrelin acetate use for a transgender-related diagnosis, including a tenfold increase in the annual number of implants from 2014 to 2016, while the number of implants placed for CPP essentially remained unchanged (Table 1) of the 92 transgender patients identified, there were 39 natal females, 52 natal males, and one patient whose natal sex was unknown. The average age at the time of implant placement for transgender children was 14 years (range 8.8 to 18.8 years), whereas the average among children with CPP was 8.5 years (range 1 to 16.8 years). There were more treated transfemales. Compared to transmales, transfemales were more likely to have implants paced at an older age (62% transfemales/natal males versus 50% transmales/natal females individuals were >13 years; P < 0.04).
Transgender Dx
2004
2005
2006
2007
2008
2009
2010
2011
2012
2013
2014
2015
2016
Natal Sex
F
0
0
0
0
0
0
0
2
1
4
5
10
24
M
0
0
0
0
0
0
1
1
1
1
1
14
38
Unkonwn
0
0
0
0
0
0
0
0
0
0
0
0
1
Age at Implant
Median age (range)
NA
NA
NA
NA
NA
NA
13.5 (NA)
10.9 (10.5- 11.3)
12.4 (11.8- 12.9)
13.3 (12.3- 16.7)
14.1 (12.0- 15.6)
14.7 (10.2- 17.8)
14 (8.8- 18.8)
Ethnicity
Hispanic
0
0
0
0
0
0
0
0
1
1
1
1
5
American Indian
0
0
0
0
0
0
0
0
0
0
0
0
0
Asian
0
0
0
0
0
0
0
0
0
0
0
1
3
Black
0
0
0
0
0
0
0
1
0
2
1
0
4
Pacific Islander
0
0
0
0
0
0
0
0
0
0
0
0
0
Other
0
0
0
0
0
0
1
1
0
0
1
7
9
White Non-Hispanic
0
0
0
0
0
0
0
1
1
2
2
12
42
Insurance
Commercial
0
0
0
0
0
0
0
2
1
5
4
18
47
Medicaid
0
0
0
0
0
0
0
1
1
0
2
5
16
Military
0
0
0
0
0
0
0
0
0
0
0
0
0
Other/self-pay
0
0
0
0
0
0
0
0
0
0
0
0
0
Hospitals placing implants -no
0
0
0
0
0
0
1
2
2
3
4
7
9
Pts receiving implants - no.
0
0
0
0
0
0
1
3
2
5
6
21
63
Precocious Puberty Dx
2004
2005
2006
2007
2008
2009
2010
2011
2012
2013
2014
2015
2016
Natal Sex
F
3
15
11
10
52
126
175
267
336
383
451
429
427
M
0
3
6
5
10
31
37
55
83
82
91
74
85
Age at Implant
Median age (range)
8 (7.1- 8.7)
7.2 (1.7- 9.8)
7.3 (3.9- 10.4)
8.1 (4.4- 11.9)
8.3 (3.4- 11.7)
8.6 (1.1- 13.1)
8.6 (1- 14.5)
8.3 (1.8- 14.5)
8.8 (1.4- 16.1)
8.7 (1.0- 14.8)
8.69 (0.9- 13.1)
8.9 (2.2- 16.8)
8.9 (1.6- 15.1)
Ethnicity
Hispanic
0
4
2
0
3
14
33
49
94
92
111
75
86
American Indian
0
0
0
0
0
0
0
0
1
2
5
5
1
Asian
0
0
0
0
1
3
5
8
12
18
17
18
19
Black
0
0
0
3
10
26
36
72
94
109
135
123
128
Pacific Islander
0
0
0
0
1
0
0
1
0
0
1
2
2
Other
3
14
15
2
13
21
35
41
43
52
68
49
42
White Non-Hispanic
0
0
0
10
34
93
103
151
175
192
205
231
234
Insurance
Commercial
0
0
0
11
38
106
124
160
201
215
246
246
284
Medicaid
0
0
0
4
14
46
85
147
193
242
287
238
213
Military
0
0
0
0
0
0
1
6
10
7
8
14
12
Other/self-pay
3
18
17
0
10
5
2
9
15
1
1
5
3
Hospitals placing implants -no
1
2
2
2
8
15
20
22
23
25
30
31
29
Pts receiving implants - no.
3
18
17
15
62
157
212
322
419
465
542
503
512
Table 1: Trends in the Use of Histrelin Acetate in Children in the United States from 2004 to 2016.
The majority of children receiving implants for a transgender related diagnosis were white non-Hispanic (white: 60, minority: 21). When compared to the distribution of patients treated for precocious puberty (white: 1428, minority: 1421), white non-Hispanic patients were more likely to be treated with a histrelin acetate implant for a transgender related diagnosis than minority patients (p<0.001). This disparity was present even among minority patients with commercial insurance (p<0.001).
From 2015 to 2016, the proportion of histrelin acetate implants used in pediatric patients with a transgender-related diagnosis markedly increased relative to the total number of implants placed for either precocious puberty or a transgender-related diagnosis (Figure 1).
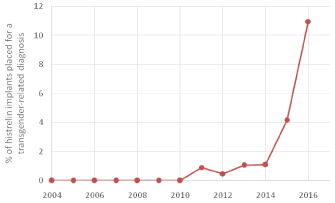
Figure 1: Percent of histrelin implants placed in children for a transgenderrelated
diagnosis relative to the total number of implants placed for either
precocious puberty or a transgender-related diagnosis.
Discussion
This study shows that the use of histrelin acetate implants in the United States for a transgender-related diagnosis has been increasing, most notably from 2014 to 2016 during which the absolute number of implants placed for CPP has remained relatively stable. These patients were more likely to be white when compared to the larger cohort of patients being treated with histrelin or leuprolide for CPP, thus identifying a potential racial disparity in access to care. This disparity may be especially relevant given recent published data from the Williams Institute suggesting a statistically higher prevalence of transgender individuals identifying as Black, Hispanic, and Native American communities than as white [9].
Natal males were more likely to receive implants at an older age compared to natal females, which may be attributable to the relative delay (1-2 years) of male pubertal onset compared to female. Moreover, our finding that the number of treated natal males is higher than the number of treated natal females is consistent with the fact that inhibiting testosterone is difficult, and GnRH analogue therapies are the only truly effective medical treatment to do so.
Given the safety and efficacy of GnRHa’sin the treatment of CPP, it is understandable that their use has been extended to include the growing needs of the pediatric transgender population. In addition to preventing the irreversible phenotypic changes that make future cross hormone therapy less effective, the use of puberty blockers may also improve the mental health of transgender adolescents. According to a recent study, transgender youth are at a much higher risk for mental disorders, including anxiety, depression, suicidal thoughts and selfharm; they are more than twice as likely as non-trans youth to be diagnosed with depression (50.6% versus 20.6%) or anxiety (26.7% versus 10%) [10]. Providing children with puberty suppression may lessen the symptoms of gender dysphoria, a DSM-5 diagnosis of significant ongoing distress, with the feeling of being assigned the wrong gender at birth [5,7,11].
A number of organizations have issued guidelines for the treatment of gender dysphoria that include puberty suppression. A Clinical Practice Guideline for the endocrine treatment of gender-dysphoric/ gender-incongruent persons, published September 2017 suggests clinicians begin pubertal hormone suppression in transgender youth once they have exhibited physical changes of puberty (i.e. Tanner stage 2/3) [12]. The World Professional Association for Transgender Health Standards of Care for the treatment of patients with gender dysphoria also includes puberty suppression for transyouth who experience worsening gender dysphoria with natal pubertal changes. Use of these medications for this purpose is still considered off-label, and in 2009, the American Academy of Pediatrics published a consensus statement on the use of GnRH analogues (GnRHa) in children, noting that the use of these therapies in children for conditions other that precocious puberty required further investigation and could not be recommended routinely; however, it did not specifically address their use in transgender children. Overall, management of gendernonconforming youth remains controversial, with a key factor being that guidelines are based primarily on expert opinion rather than scientific data.
While data on the use of puberty blockers is scarce, in the past decade or so, it is believed that thousands of transgender children and their families have chosen to intervene medically in order to delay puberty, and evidence points to an ever-growing patient population. In one of the largest pediatric medical centers, researchers reported the number of individuals who presented with gender identity issues rose from an average of 4.5 patients per year from 1998 and 2006, to an average of 19 patients per year from 2007 to 2009 [13]. In a more recent study from 2016, physicians at another large pediatric endocrinology clinic reported a “dramatic increase” in referrals for gender dysphoria since 2002, finding that of the 38 patients referred between 2002 and 2015, 74% were referred during the last 3 years despite no changes in the referral base [14]. Studies from outside the United States are reporting similar trends [15]. For example, in the UK, the number of children referred to gender identity clinics has increased from 98 referrals in 2009-2010 to 1,986 referrals in 2016- 2017 [16].
As the size of the pediatric transgender population seeking medical intervention grows, it becomes increasingly important to conduct formal epidemiologic studies exploring not only the prevalence and incidence of gender nonconforming youth but, importantly, their access to care. Sex hormone blocking therapies can cost thousands of dollars per month. As a result, it is estimated that many adolescent transgender patients deemed appropriate candidates for medical intervention, but live in a state where transgender care is not covered by insurance, are unable to obtain the treatment due to unaffordability. The National Center for Transgender Equality published a report in 2016 noting that 31 states have no policy ensuring private insurance or Medicaid coverage of trans health care; 13 states have mandated coverage for trans healthcare; and four have some protection – a policy banning trans exclusions in either private insurance or Medicaid – but not both [17]. In one clinical cohort, it was estimated that insurance had covered blockers for <20% of patients since 2007 [18]. Lupron-Depot (Ped) 3-month injections cost more than $6000; the pediatric brand of the Histrelin acetate implant, Supprelin, costs more than $25,000 (costs are approximate). Medicaid patients may be particularly at risk for having limited access to these therapies. In most states, Medicaid has no platform for authorizing the use of Histrelin implants for the management of a transgender-related diagnosis and a recent study has demonstrated that overall, disparities exist in access to specialty pediatric services between privately insured and government insured patients [19]. The first step in addressing barriers to care may be to quantify utilization of medical therapies in order better understand need.
Conclusion
In summary, the use of sex hormone blocking implants in transgender children in the United States has increased nearly twentyfold from 2014 to 2016, accounting for >10% of their use in 2016. Strategies appear to be warranted to perform ongoing epidemiologic surveillance in order to better gauge the growing needs of this population, increase awareness in the medical community, and inspire long-term outcomes research on the consequences of the offlabel uses of these medications.
References
- Radix A, Silva M. Beyond the guidelines: challenges, controversies, and unanswered questions. Pediatr Ann. 2014; 43: 145-150.
- Eugster EA, Clarke W, Kletter GB, Lee PA, Neely EK, Reiter EO, et al. Efficacy and safety of histrelin subdermal implant in children with central precocious puberty: a multicenter trial. J Clin Endocrinol Metab. 2007; 92: 1697-1704.
- Care JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, Antoniazzi F, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. 2009; 123: 752-762.
- de Vries AL, McGuire JK, Steensma TD, Wagenaar EC, Doreleijers TA, Cohen-Kettenis PT, et al. Young adult psychological outcome after puberty suppression and gender reassignment. Pediatrics. 2014; 134: 696-704.
- E C, W B, M B. Standards of Care for the Health of Transsexual, Transgender, and Gender-nonconforming people. 2011; 11: 165-232.
- Bertelloni S, Mul D. Treatment of central precocious puberty by GnRH analogs: long-term outcome in men. Asian J Androl. 2008; 10: 525-534.
- Hembree WC, Cohen-Kettenis P, Delemarre-van de Waal HA, Gooren LJ, Meyer WJ, Spack NP, et al. Endocrine treatment of transsexual persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2009; 94: 3132-3154.
- Fletcher DM. Achieving data quality. How data from a pediatric health information system earns the trust of its users. J AHIMA. 2004; 75: 22-26.
- Meyer IH, Brown TN, Herman JL, Reisner SL, Bockting WO. Demographic Characteristics and Health Status of Transgender Adults in Select US Regions: Behavioral Risk Factor Surveillance System, 2014. Am J Public Health. 2017; 107: 582-589.
- Reisner SL, Vetters R, Leclerc M, Zaslow S, Wolfrum S, Shumer D, et al. Mental health of transgender youth in care at an adolescent urban community health center: a matched retrospective cohort study. J Adolesc Health. 2015; 56: 274-279.
- Cohen-Kettenis PT, Delemarre-van de Waal HA, Gooren LJ. The treatment of adolescent transsexuals: changing insights. J Sex Med. 2008; 5: 1892-1897.
- Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, et al. Endocrine Treatment of Gender-Dysphoric/Gender- Incongruent Persons: An Endocrine Society* Clinical Practice Guideline. J Clin Endocrinol Metab. 2017; 102: 3869-3903.
- Spack NP, Edwards-Leeper L, Feldman HA, Leibowitz S, Mandel F, Diamond DA, et al. Children and adolescents with gender identity disorder referred to a pediatric medical center. Pediatrics.2012; 129: 418-425.
- Chen M, Fuqua J, Eugster EA. Characteristics of Referrals for Gender Dysphoria Over a 13-Year Period. J Adolesc Health. 2016; 58: 369-371.
- Wood H, Sasaki S, Bradley SJ, Singh D, Fantus S, Owen-Anderson, A et al. Patterns of referral to a gender identity service for children and adolescents (1976-2011): age, sex ratio, and sexual orientation. J Sex Marital Ther. 2013; 39: 1-6.
- GIDS Referrals figures for 2016/17. In: Gender Identity Development Service, National Health Service. 2017.
- James SE, Herman JL, Rankin S, Keisling M, Mottet L, Anafi M, et al. The Report of the 2015 Transgender Survey. Washington, DC: National Center for Transgender Equality. 2016.
- Edwards-Leeper L, Spack NP. Psychological evaluation and medical treatment of transgender youth in an interdisciplinary “Gender Management Service” (GeMS) in a major pediatric center. J Homosex. 2012; 59: 321-336.
- Bisgaier J, Rhodes KV. Auditing access to specialty care for children with public insurance. N Engl J Med 2011; 364: 2324-2333.