
Research Article
J Pediatri Endocrinol. 2019; 4(1): 1029.
Serum Levels and Gestational Curve of Adiponectin and Leptin During Adolescent Pregnancy
Baratto I, Daher S, Frutuoso Lobo T and Aparecida Falbo Guazzelli C*
Department of Obstetrics, Sao Paulo Federal University, Brazil
*Corresponding author: Aparecida Falbo Guazzelli C, R Bandeiras 253 ap 181- Santo Andre, Brazil
Received: April 08, 2019; Accepted: June 14, 2019; Published: June 21, 2019
Abstract
Adiponectin and leptin have a characteristic pattern and play important roles during pregnancy in adults, although little is known about them in adolescent gestation. The objective of this study was to develop a gestational curve for the weekly serum levels of adiponectin and leptin among adolescent pregnant women. Pre-gestational BMI and weight gain were also evaluated and correlated with the serum concentration of these molecules. The study evaluated adolescents with pre-gestational BMI of eutrophy during the evolution of gestation. Peripheral blood samples were collected to evaluate serum adipokine concentrations by the ELISA method. A total of 157 pregnant women participated in the study, totaling 471 blood samples. Serum levels of adiponectin showed significant differences, showing a drop in concentration during gestation (p = 0.0003), we did not observe a correlation between pre-gestational BMI, weight gain and serum levels (p = 0.36; p = 0.10, respectively). With the advancement of gestation, we identified an increase in serum leptin levels (p ‹0.0001), a positive correlation between serum levels and pre-gestational BMI and also between weight gain (p = 0.003; p = 0, 0007, respectively). We conclude that adiponectin decreases with the evolution of pregnancy, however it has no correlation with BMI and weight gain and leptin increases during pregnancy presenting a direct correlation with BMI and weight gain. The pattern of adiponectin and leptin production observed in adolescent pregnant women is similar to that seen in adult pregnant women.
Keywords: Leptin; Adiponectin; Adolescent; Pregnancy; Adipokine
Introduction
Adipokines are proteins secreted by adipose tissue, described as molecules with diverse physiological structures and functions [1,2]. They act as classical cytokines, growth factors and complementary systemic proteins, are involved in the regulation of blood pressure, vascular homeostasis, angiogenesis, lipid and glycemia metabolism [3]. Adiponectin and leptin are the most abundant adipokines synthesized by adipose tissue [4].
Adiponectin exhibits antihyperglycemic, antiatherogenic and anti-inflammatory properties, promotes insulin sensitization, decreases the hepatic production of glucose and increases the action of insulin in the liver [5-7]. Serum levels may vary according to sex, being higher in women [8,9]. Research does not indicate that this difference also occurs with adolescents, but they report lower concentrations between the pubescent and obese [10]. At puberty the levels are significantly lower than those observed in the pre-pubertal period, this is presumed to be a function of the decrease in Insulin Resistance (IR) that occurs at this stage [11,12].
The serum concentration of adiponectin during pregnancy decreases with the advancement and installation of IR, returning to the pre-gravid concentration after delivery. Studies indicate that there is a negative correlation between serum levels and gestational age [5-7,13-15], it seems that during pregnancy, adiponectin acts on the energy balance, on the fatty oxidation of fatty acids, decreasing production glucose and increasing the action of insulin by the liver [13,16,17].
Leptin is involved in inflammatory processes, immune mediated responses, appetite regulation [18], energy storage, modulation of the homeostatic system and regulation of blood pressure, aid in thermogenesis, angiogenesis, insulin secretion by the pancreas, in the production of glucose by the liver and in its uptake through the muscle [19,20-22]. Its synthesis is directly related to the amount of adipose tissue present in the individual’s body, therefore, plasma levels differ in people with the same Body Mass Index (BMI) [23]. In addition to BMI, other factors such as sex, age, fasting, overeating, diet composition and hormones may influence the serum levels of this adipokine [6,7,9,10].
In adults, plasma concentration is higher in females when compared to values found in males [18,28]; in children and adolescents, leptin levels are related to changes in body composition [53-55]. In the gestational period, the major changes in plasma levels of this adipokine occur, being significantly higher [18,29,30]. During pregnancy, leptin plays a key role in regulating placental growth, nutrient transfer, angiogenesis, pulmonary maturation, and trophoblast invasion [31].
Research has shown that blood levels of adiponectin and leptin are related to physiological changes during pregnancy in adult women, but information on the behavior of these molecules in adolescent gestation so far remains unknown.
Thus, the objective of this study was to evaluate the serum levels of these molecules during the evolution of gestation in adolescents and to develop a gestational curve for weekly serum levels, and to correlate pre-gestational BMI and total weight gain of adolescents with concentrations of these adipokines.
Materials and Methods
This was a prospective cohort study performed with adolescent pregnant women attended in the Adolescent Prenatal Sector of the Paulista School of Medicine from February 2013 to March 2018. The study was submitted to the Research Ethics Committee of the Federal University of São Paulo / Hospital São Paulo, being approved under the consubstantiated opinion nº 1514/11. Included in this study were adolescent eutrophic pregnant women who were being followed in the prenatal sector of adolescents of Escola Paulista de Medicina (EPM) - UNIFESP.
All patients who accepted to participate in the study spontaneously were included in the sample after reading, understanding and signing the informed consent form.
The patients were followed during gestational development, and blood samples were collected during pregnancy (3 samples, one in each trimester). Pregnant women, older than 10 years and younger than 20 years [32], who presented with pre-gestational BMI of eutrophy (18.5-24.9 kg/m²) were included in this study [33]. Those with multiple gestation, or on the use of corticosteroids, antibiotics, immunosuppressant’s and / or anti-inflammatories, and those that evolved with some clinical intercurrence during pregnancy progression, such as gestational diabetes, preeclampsia, intrauterine growth restriction, or (hypertension, diabetes mellitus, systemic lupus erythematosus, rheumatoid arthritis, rheumatic fever and asthma). BMI was calculated based on the pre-gestational weight and height, being mentioned by the pregnant woman at the first prenatal visit, the nutritional diagnosis was determined according to the Institute of Medicine (IOM) (2009) [33]. Gestational weight gain was determined by the difference between the weight of the patient at the last predelivery visit and the pre-gestational weight.
We collected and stored in dry tube, 8 mL of blood by venipuncture, and serum levels of adiponectin and leptin were evaluated by the ELISA method, and the plasma values of the molecules expressed in ng/mL (nanograms / milliliters) were determined.
The normality tests Skewness and Kurtosis, Kolmogorov-Smirnov and Shapiro-Wilk were applied to evaluate the distribution of the quantitative variables. For analysis of variance between the groups the One-Way ANOVA test was applied to measure those of parametric distributions and the Kruskal-Wallis test was used to analyze the non-parametric ones, followed by Tukey post-tests or Dunn posttest, respectively. For the analysis of categorical variables the chisquare test was adopted. The Pearson test was applied to calculate the correlation coefficients. The level of significance was set at p ‹0.05. Statistical analyzes were performed using standard software (GraphPad Prism, v6.0 for Windows). As we did not find previous studies that allowed to calculate the sample size recommended for this investigation, the proposal was to carry out a study that serves as the basis for future research. Thus, the sample was of convenience, including all the patients attended in the study period who were in agreement with the established parameters.
Results
The study included 157 healthy adolescent pregnant women, totaling 471 blood samples collected between the 9th and 39th gestational week.
The age of the pregnant women included in this study ranged from 12 to less than 20 years, with an average of 16.51 years (standard deviation 1.76), and there was no significant difference between patients grouped by gestational age (p = 0.06; ANOVA One-Way Test). Regarding the race / color of the patients included, there was a statistical difference between those grouped by gestational age (p ‹0.0001; Chi-Square test). The sample included 81 collections of blood from brown adolescents and 76 collections of blood in white adolescents.
The pre-gestational BMI was 22 kg/m2 (Interquartile Interval 21,40 - 22,50), presenting a significant difference between the patients included in the study grouped by gestational age (p = 0.004; Kruskal- Wallis test).
We also identified statistical difference regarding smoking and alcohol variables (p ‹0.0001, Chi-square test, both). Our results showed that 4.9% (n = 23) of the sample studied had smoking during gestation and 2.3% (n = 11) of ingesting alcohol.
With the evolution of pregnancy, serum levels of adiponectin presented significant differences, characterized by a drop in concentration (p = 0.0003; Kruskal-Wallis test). The data are presented in (Table 1 and Figure 1).
Gestational Week
N
Minimum
25% Interquartile Interval
Medium
75% Interquartile Interval
Maximum
9ª
16
1726
2047
3473
4331
6282
10ª
16
1141
2507
3733
5267
7295
11ª
15
1350
2596
3786
6389
7575
12ª
15
1058
2891
3677
5263
6924
13ª
18
2392
2958
3578
4442
8879
14ª
16
1507
1570
3149
3823
5690
15ª
15
1258
1913
2635
6579
14284
16ª
15
1001
1514
2569
6250
10943
17ª
15
1205
2447
3735
5690
8377
18ª
15
1205
2476
3781
4483
6579
19ª
15
1575
2964
3835
7362
9697
20ª
15
1322
1599
2998
3459
5960
21ª
15
1526
2224
3520
5784
10943
22ª
15
1579
2152
3369
4954
9697
23ª
15
1569
1632
2363
5236
5471
24ª
15
1233
3349
4183
6940
8520
25ª
15
1511
2569
3120
4250
6199
26ª
15
1540
1789
2286
4445
5391
27ª
15
1278
2157
3292
3697
5541
28ª
15
1353
2458
3520
4723
8588
29ª
15
1036
1599
2362
3261
5367
30ª
15
1169
1838
2372
3336
4445
31ª
15
1247
1831
2534
3687
7287
32ª
15
1007
1951
2326
3372
6352
33ª
15
1252
1568
2160
2470
5037
34ª
15
1158
1336
2564
3569
5348
35ª
15
1506
1800
2963
3185
22103
36ª
15
1621
1883
2610
4789
9056
37ª
15
1036
1659
1931
3466
9754
38ª
15
1509
1943
2964
3233
5031
39ª
15
1504
1863
2275
3103
3589
Kruskal-Wallis test, p=0,0003.
Table 1: Adiponectin serum concentrations (ng/mL) during the gestational weeks of adolescents attended in the prenatal sector of adolescents of Escola Paulista de Medicina - UNIFESP.
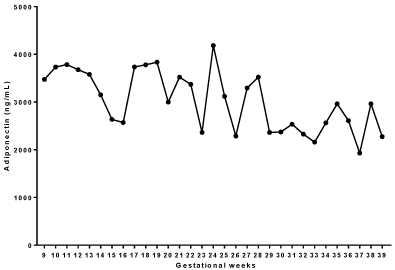
Figure 1: Adiponectin serum levels (ng/mL) during the gestational weeks of
adolescents attended in the prenatal sector of adolescents of Escola Paulista
de Medicina - UNIFESP.
We did not observe a correlation between the pre-gestational BMI, the weight gain of the pregnant women and the serum levels of adiponectin (p = 0.36, p = 0.10, respectively).
After the Kruskal-Wallis test, we observed that there were no statistical differences in serum adiponectin levels between the gestational weeks of the adolescents included in the study (Dunn’s post-test).
With the advancement of gestational weeks, we identified an increase in serum leptin levels (p ‹0.0001; One Way Test-ANOVA). The results are described in (Table 2 and Figure 2).
Gestational Week
N
Minimum
Maximum
Average
Standard Deviation
9ª
16
9,6
59,75
24,29
14,83
10ª
16
9,57
45,9
22,11
10,09
11ª
15
11,29
98,01
38,46
30,29
12ª
15
5,83
95,65
40,10
32,56
13ª
18
11,48
103,3
29,91
22,45
14ª
16
5,59
54,48
26,12
15,27
15ª
15
2,15
65,16
24,51
16,51
16ª
15
5,29
82,23
32,91
22,24
17ª
15
7,74
43,74
18,47
10,69
18ª
15
7,73
38,06
19,54
8,867
19ª
15
8,84
55,34
28,1
11,16
20 ª
15
11,56
96,91
38,42
23,87
21ª
15
12,85
54,45
28,34
11,01
22ª
15
12,15
54,46
24,37
11,97
23ª
15
7,18
55,45
33,53
15,64
24ª
15
3,42
46,22
20,01
13,26
25ª
15
4,76
61,51
21,84
17,05
26ª
15
11,7
48,05
31,00
12,96
27ª
15
4,47
51,06
26,15
13,98
28ª
15
1,31
40,67
24,89
11,11
29ª
15
11,05
69,42
31,59
16,25
30ª
15
4,67
87,76
39,67
23,52
31ª
15
11,88
83,12
40,42
23,78
32ª
15
4,49
77,36
39,57
22,15
33ª
15
11,04
160,2
39,30
39,22
34ª
15
7,56
68,42
30,62
17,49
35ª
15
12,38
85,75
45,52
21,32
36ª
15
2,87
77,51
42,39
22,37
37ª
15
14,17
79,61
40,15
16,93
38ª
15
19,63
85,75
49,93
22,51
39ª
15
11,07
85,5
34,75
20,57
ANOVA One-Way Test, p <0,0001.
Table 2: Serum leptin concentrations (ng/mL) during the gestational weeks of adolescents attended in the prenatal sector of adolescents of the Escola Paulista de Medicina - UNIFESP.
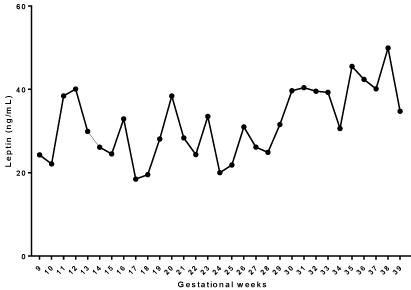
Figure 2: Serum levels of Leptin (ng/mL) during the gestational weeks of
adolescents attended in the prenatal sector of adolescents of Escola Paulista
de Medicina - UNIFESP.
After the One-Way ANOVA test, we observed statistical differences regarding serum leptin levels between some gestational weeks (compared to two, Tukey’s post-test). We identified differences in the comparisons between the 17th to the 38th, the 18th to the 38th, the 24th to the 38th and the 25th to the 38th.
We identified a positive correlation between pre-gestational BMI and the weight gain of pregnant women with serum leptin levels (p = 0.003, p = 0.0007, respectively).
Discussion
This study performed with adolescent pregnant women evaluated the serum profile of adiponectin (ng/mL) and leptin (ng/mL) during the course of pregnancy (9th to 39th gestational weeks). Through the values found, a gestational curve was developed with the serum concentrations for these molecules.
In pregnancy, adiponectin acts on the development of the inflammatory response in the maternal-fetal unit. This reaction is physiological and needs to be modulated so that an adequate evolution of the pregnancy occurs. The involvement of this protein in local and systemic inflammatory reactions is already well known [34].
The literature indicates that this molecule presents specific behavior in pregnant women, but, unlike other adipokines, its serum concentration tends to reduce with the progression of gestation [13,17,35,36]. There appears to be a negative correlation between adiponectin and gestational age [14,15]. Similar results were observed in our study with adolescents.
Some authors have reported that plasma levels of adiponectin decrease gradually with the advancement of pregnancy because its concentration reduces as the fat tissue increases [13-15,37], but these values return to normal soon after delivery [13]. Studies have reported that the serum concentration of adiponectin at certain periods of gestation in healthy women may be altered from the first trimester [36-38]. Some studies indicate a correlation between IR and adiponectin concentration in adult pregnant women [39-42].
In similar studies, researchers demonstrated significant differences during pregnancy progression in adults, where they observed a reduction in the serum concentration of adiponectin when they compared the first to the third trimester [6,37,39,43].
Our results show that the behavior of adiponectin in the evolution of gestation in adolescents is similar to what the literature indicates in pregnancy for adults.
When we observed the serum levels of adiponectin (ng/mL) during gestational weeks we found that the concentration of this molecule tends to be higher at the beginning of gestation (median 3473 ng/mL) than at the end of pregnancy (median 2275 ng/mL).
In this study, we observed a peak serum adiponectin concentration at the 24th gestational week. Our hypothesis is that this alteration is related to the installation of IR, however we do not find other studies that support this hypothesis in the literature.
The literature shows that, unlike other hormones secreted by adipose tissue, serum adiponectin levels decrease as adiposity increases, being inversely correlated with obesity, IR and metabolic syndrome [2,44].
In relation to leptin, our study showed a significant increase (p = 0.0001) with the evolution of pregnancy, suggesting that this change can be gradually observed. The literature shows that plasma concentrations of leptin increase during pregnancy in adults, concluding that this is a condition in which hyperleptinemia is observed [18,45-47].
Researchers studying serum levels of leptin in adult pregnant women found that the plasma concentration had a significant increase between the first and third trimester of gestation with postpartum decline [46]. During pregnancy, the concentration of leptin in maternal serum is higher than that observed in non-pregnant women [29].
Our results were similar to those presented in recent studies with adult pregnant women [45,49,50]. Serum levels of leptin present changes during pregnancy, this increase occurs in pregnant women with and without obstetric complications. Elevation in serum concentration during the course of pregnancy has been extensively investigated with the main purpose of elucidating a possible relationship to clinical intercurrences such as preeclampsia, gestational diabetes, intrauterine growth restriction and low birth weight [19,28,45,46,51-55].
Studies have shown that serum leptin concentration during pregnancy with clinical complications appears to be related to hyperleptinemia [28,45,46,51-55], and that women who experience spontaneous or recurrent miscarriage appear to have lower plasma levels such a molecule [56,57].
Our results are similar to those observed in gestation of adult women [45,52]. Serum leptin concentrations begin to rise from the onset of pregnancy and are higher than that of non-pregnant women throughout pregnancy [23].
The literature indicates that leptin values are directly related to the amount of adipose tissue present in the body, since the plasma concentration differs in people with the same BMI [23].
It is known that gestation is characterized by metabolic and endocrine adaptations of the maternal organism, including increased body weight and adipose tissue. Thus elevated serum leptin levels during pregnancy are related to weight gain and BMI, as well as changes in hormone levels that may stimulate the secretion of this molecule (eg, insulin) [59]. Our results are similar to those performed with adult pregnant women, where a positive correlation was observed between maternal plasma leptin concentration and anthropometric data at both the beginning and the end of gestation. This association suggests that body weight and, probably, adiposity gain are important factors for the increase in circulating leptin levels [58,60]. Thus, based on our study we can affirm that in adolescent pregnant there is an increase in the plasma concentration of leptin and reduction in adiponectin during the course of gestation. In adolescents we observed similar results to those observed for the adult pregnant woman, thus showing that these molecules have the same behavior independent of the age group. The continuity of this research may contribute to elucidate the doubts about the behavior of adipokines in the progression of gestation, but it is worth noting that until now the literature does not present a gestational curve with the values determined for adults and/or adolescents.
Conclusion
With the evolution of pregnancy, serum levels of adiponectin (ng / mL) presented a reduction in blood concentration, with significant differences (p = 0.0003). When we compared the serum values of adiponectin from two to two gestational weeks, we did not observe a statistically significant alteration, evidencing a progressive decrease. No correlation was observed between the pre-gestational BMI and the weight gain of the pregnant women and the plasma levels of adiponectin (ng/mL) (p = 0.36, p = 0.10, respectively).
With the evolution of pregnancy, serum levels of leptin (ng/ mL) showed an increase in blood concentration, with significant differences (p ‹0.0001). The serum concentration of leptin (ng/mL) presented statistically significant differences when we compared the 17th, 38th, 18th, 38th, 24th, 38th and 25th, 38th. We observed a positive correlation between the pre-gestational BMI and the weight gain of the pregnant women and the serum concentration of leptin (ng/mL) (p = 0.003; p = 0.0007, respectively).
The pattern of adiponectin and leptin production observed in adolescent pregnant women is similar to that seen in adult pregnant women.
Acknowledgements
Author contributions
Indiomara Baratto – Performed the laboratories tests, collected the data, collaborated in patient recruitment, made clinical contribuitions, wrote the manuscript.
Silvia Daher – designed the study analyzed the data and performance the statistical analysis
Thalita Frutuoso Lobo – performed the laboratories tests and the statistical analysis
Cristina Aparecida Falbo Guazzelli – designed the study, collected the data, collaborated in patient recruitment, wrote and corrected the manuscript.
References
- Trayhurn P. Adipocyte Biology. Obesity. 2007; 8: 41-44.
- Kennedy GC. The Role of Depot Fat in the Hypothalamic Control of Food Intake in the Rat. Proc R Soc Lond B Biol Sci. 1953; 140: 578-592.
- Trayhurn P, Wood S. Adipokines: inflammation and the pleiotropic role of white adipose tissue. J of Nutrition. 2004; 92: 347-355.
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AWJ. Obesity is associated with macrophage accumulation in adipose tissue. The J of Clinical Investigation. 2003; 112: 1796-1808.
- Tovi SM, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F, et al. Adiponectin Multimers in Normal Pregnancy. J Matern Fetal Neonatal Med. 2008; 21: 796-815.
- Vitoratos N, Valsamakis G, Mastorakos G, Boutsiadis A, Salakos N. Kouskoni et al. Pre and Early Post-Partum Adiponectin and Interleukin-1 Beta Levels in Women With and Without Gestational Diabetes. Hormones. 2008; 7: 230- 236.
- Low FC, Tohit ERM, Chong PP, Idris F. Adiponectin SNP45TG is Associated with Gestational Diabetes Mellitus. Arch Gynecol Obstet. 2011; 283: 1255- 1260.
- Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired Multimerization of Human Adiponectin Mutants Associated with Diabetes. J Biol Chem. 2003; 278: 40352-40363.
- Bidulesco A, Liu J, Hickson DA, Hairston KG, Fox ER, Arnett DK, et al. Gender differences in the association of visceral and subcutaneous adiposity with adiponectin in African Americans: the Jackson Heart Study. Cardiovascular Disorders. 2013; 13: 1471-2261.
- Boyne MS, Bennett NR, Cooper RS, Royal-Thomas TY, Bennett FI, Luke A, et al. Sex-differences in adiponectin levels and body fat distribution: Longitudinal observations in Afro-Jamaicans. Diabetes Research and Clinical Practice. 2010; 90; 33-36.
- Andersen KK, Frstyk J, Wolthers Od, Heuck C, Flyvbjerg A. Gender Differences of Oligomers and Total Adiponectin During Puberty: A Cross- Sectional Study of 859 Danish School Children. J Clin Endocrinol Metab. 2007; 92: 185718-185762.
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic Syndrome in Childhood: Association With Birth Weight, Maternal Obesity, and Gestational Diabetes Mellitus. Pediatrics. 2005; 115: 290-296.
- Zavalza-Gómez AB, Anaya-Prado R, Rincón-Sánchez AR, Mora-Martínez JM. Adipokines and Insulin Resistance during Pregnancy. Diabetes Res Clin Pract. 2008; 80: 8-15.
- Hara K, Yamauchi T, Kadowaki T. Adiponectin: An Adipokine Linking Adipocytes and Type 2 Diabetes in Humans. Curr Diab Rep. 2005; 5: 136- 140.
- Nanda S, Savvidou M, Syngelaki A, Akolekar R, Nicolaides KH. Prediction of Gestacional Diabettes Mellitus by Maternal Factors and Biomarkers at 11 to 13 Weeks. Prenat Diagn. 2011; 31: 135-141.
- Tartaglia LA, Dembski BM, Weng X, Deng N, Culpepper J, Devos R, et al. Identification and expression cloning of a leptin receptor (Ob-R). Cell. 1995; 83: 1263-1271.
- Valsamakis G, Kumar S, Creatsas G, Mastorakos G. The Effects of Adipose Tissue and Adipocytokines in Human Pregnancy. Ann NY Acad Sci. 2010; 1205: 76-81.
- Highman TJ, Friedman JE, Huston LP, Wong WW, Catalano PM. Longitudinal Changes in Maternal Serum Leptin Concentration, Body Composition, and Resting Metabolic Rate in Pregnancy. Am J Obstet Gynecol. 1998; 178: 1010-1015.
- Zhang Y, Proença R, Maffei M, Barone M, Leopold L, Friedman JM. Positional Cloning of the Mouse Obese Gene and its Human Homologue. Nature. 1994; 372: 425-432.
- Havel PJ. Mechanisms Regulating Leptin Production: Implications for Control of Energy Balance. Am J Clin Nutr. 1999; 70: 305-306.
- Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central Nervous System Control of Food Intake. Nature. 2000; 404: 661-671.
- La Cava A, Matarese G. The Weight of Leptin in Immunity. Nat Rev Immunol. 2004; 4: 371-379.
- Moreno LA, Pineda I, Rodriguez G, Fleta J, Giner A, Juste MG, et al. Leptin and Metabolic Syndrome in Obese and Non-Obese Children. Horm Metab Res. 2002; 34: 394-399.
- Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR, et al. Responses of Leptin to Short-Term Fasting and Refeeding in Humans. Diabetes. 1996; 45: 1511-1515.
- Jenkins AB, Markovic TP, Fleury A, Campbell LV. Carbohydrate Intake and Short-Term Regulation of Leptin in Humans. Diabetologia. 1997; 40: 348- 351.
- Mantzoros CS, Prasad AS, Beck FWJ, Grabowski S, Kaplan J, Adair C, et al. Zinc May Regulate Serum Leptin Concentration in Humans. J Am Coll Nutr. 1998; 17: 270-275.
- Wabitsch M, Jensen PB, Blum WF, Christoffersen CT, Englaro P, Heinze E, et al. Insulin and Cortisol Promote Leptin Production in Cultured Human Fat Cells. Diabetes. 1996; 45: 1435-1438.
- Henson MC, Castracane VD. Leptin in Pregnancy: An Uptade. Bio Reprod. 2006; 74: 218-229.
- Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, et al. Nonadipose Tissue Production of Leptin: Leptin as Anovel Placenta Derived Hormone in Humans. Nat Med. 1997; 3: 1029-1033.
- Tomimatsu T, Yamaguchi M, Murakami T, Ogura K, Sakata M, Mitsuda N, et al. Increase of Mouse Leptin Production by Adipose Tissue after Midpregnnacy Gestational Profile of Serum Leptin Concentration. Biochem Biophys Res Commun. 1997; 240: 213-215.
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S. Leptin Activates Anorexigenic POMC Neurons through a Neural Network in the Arcuate Nucleus. Nature. 2001; 411: 480-484.
- World Health Organization (OMS). Adolescent health. 2013.
- National Research Council/Institute of Medicine (NRC/IOM). Weight gain during pregnancy: Reexamining the guidelines. Washington (DC): National Academies Press. 2009.
- Moulin CM, Marguti I, Peron JP, Rizzo LV, Halpern A. Impact of adiposity on immunological parameters. Arq Bras Endocrinol Metabol. 2009; 53: 183-189.
- Sagawa N, Yura S, Itoh H, Mise H, Kakui K, Korita D, et al. Role of Leptin in Pregnancy - A Review. Am J of Obstetrics and Gynecology. 2006; 194: 1537-1545.
- Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Plasma Adiponectin Concentrations In Non Pregnant, Normal Pregnancy And Overweight Pregnant Women. J Perinat Med. 2007; 35: 522-531.
- Fuglsang J, Skjaerbaek C, Frystyk C, Flyvbjerg A, Ovesen P. A Longitudinal Study of Serum Adiponectin during Normal Pregnancy. BJOG. 2006; 113: 110-113.
- Spadafranca A, Piuri G, Bulfoni C, Liguori I, Battezzati A, Bertoli S, et al. Adherence to the Mediterranean Diet and Serum Adiponectin Levels in Pregnancy: Results from a Cohort Study in Normal Weight Caucasian Women. Nutrients. 2018; 20: 10.
- Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in Human Pregnancy: Implications for Regulation of Glucose and Lipid Metabolism. Diabetologia. 2006; 49: 1677-1685.
- Lopez-Bermejo A, Fernandez-Real JM, Garrido E, Rovira R, Brichs R, Genaro P, et al. Maternal Soluble Tumour Necrosis Factor Receptor Type 2 (sTNFR2) and Adiponectin are Both Related to Blood Pressure during Gestation and Infant’s Birthweight. 2004; 61: 544-552.
- McLachlan KA, O’Neal D, Jenkins A, Alford FP. Do Adiponectin, TNF Alpha, Leptin and CRP Relate to Insulin Resistance in Pregnancy? Studies in Women With and Without Gestational Diabetes, During and After Pregnancy. 2006; 22: 131-138.
- Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced Adiponectin Concentration in Women With Gestational Diabetes: A Potential Factor in Progression to Type 2 Diabetes. 2004; 27: 799-800.
- Hedderson MM, Darbinian J, Havel PJ, Quesenberry CP, Sridhar S, Ehrlich S, et al. Low Prepregnancy Adiponectin Concentrations are Associated With a Marked Increase in Risk for Development of Gestational Diabetes Mellitus. Epidemiology. 2013; 36: 3930-3937.
- Hauner H. The New Concept of Adipose Tissue Function. Physiol Behav. 2004; 83: 653-658.
- Vahamiko S, Isolauri E, Laitinen K. Weight Status and Dietary Intake Determine Serum Leptin Concentrations in Pregnant and Lactating Women and Their Infants. Br J Nutr. 2013; 110: 1-9.
- Anim-Nyame N, Sooranna SR, Steer PJ, Johnson MR. Longitudinal Analysis of Maternal Plasma Leptin Concentrations During Normal Pregnancy and Pre-eclampsia. Hum Reprod. 2000; 15: 2033-2036.
- Hardiel L, Trayhurn P, Abramovich D, Fowler P. Circulating Leptin in Women: A Longitudinal Study in the Menstrual Cycle and During Pregnancy. Clin Endocrinol. 1997; 47: 101-106.
- Meyer BJ. Maternal obesity is associated with the formation of small dense LDL and hypoadiponectinemia in the third trimester. J Clin Endocrinol Metab. 2013; 169: 643-652.
- Misra VK, Straughen JK, Trudeau S. Maternal Serum Leptin During Pregnancy and Infant Birth Weight: the Influence of Maternal Overweight and Obesity. Obesity. 2013; 21: 1064-1069.
- Filho DSC, Correa JOA, Ramos PS, Montessi MO, Aarestrup BJV, Aarestrup FM. Body Weight Gain and Serum Leptin Levels of Non Overweight and Overweight/Obese Pregnant Women. Med Sci Monit. 2013; 19: 1039-1043.
- Briana DD, Puchner AM. Adipocytokines in Normal and Complicated Pregnancies. Rep Sci. 2009; 16: 921-937.
- Saylan F, Koken G, Cosar E, Saylan A, Arioz T, Sahin F, et al. Maternal and Fetal Leptin and Ghrelins Levels: Relationship With Fetal Growth. Arch Gynecol Obstet. 2011; 284: 327-329.
- Al-Atawi FS, Addar MH, Warsy AS, Babay ZA. Leptin Concentration During Different Trimesters of Pregnancy and its Relation to Other Pregnancy Hormones. Saudi Med J. 2004; 25: 1617-1622.
- Hardiel L, Trayhurn P, Abramovich D, Fowler P. Circulating Leptin in Women: A Longitudinal Study in the Menstrual Cycle and During Pregnancy. Clin Endocrinol. 1997; 47: 101-106.
- Sivan E, Whittaker PG, Sinha D, Homoko CJ, Lin M, Reece EA, et al. Leptin in Human Pregnancy: The Relationship with Gestational Hormones. Am J Obstet Gynecol. 1998; 179: 1128-1132.
- Lage M, Mayor RVG, Tomé MA, Cordido F, Valle-Inclan F, Considine RV, et al. Serum Leptin Levels in Women Throughout Pregnancy and the Postpartum Period and in Women Suffering Spontaneous Abortion. Clin Endocrinol. 1999; 50: 211-216.
- Tommaselli GA, Di Spiezio A, Di Carlo C, Bifulco G, Cerrota G, Cirillo D, et al. Do Serum Leptin Levels Have a Role in the Prediction of Pregnancy Outcome in Case of Threatened Miscarriage? Clin Endocrinol. 2006; 65: 772-775.
- Maple-Brown. Maternal pregravid weight is the primary determinant of serum leptin and its metabolic associations in pregnancy, irrespective of gestational glucose tolerance status. J Clin Endocrinol Metab, 2012; 97: 4148-4155.
- Caprio M, Fabbrini E, Isidori AM, Aversa A, Fabbri A. Leptin in reproduction. Trends Endocrinol Metab. 2001; 12: 65-72.
- Kiess W. Adipocytes and adipose tissue. Best Pract Res Clin Endocrinol Metab. 2008. 22: 135-153.