
Research Article
J Pediatri Endocrinol. 2019; 4(1): 1030.
Hearing Loss Severity and Progression in Children with Congenital Hypothyroidism
Inglesby DC1, Sluder CE1*, Liu YF2, Nguyen SA2 and Meyer TA2
¹College of Medicine, Medical University of South Carolina, USA
²Department of Otolaryngology, Medical University of South Carolina, USA
*Corresponding author: Sluder CE, College of Medicine, Medical University of South Carolina, 199 Rutledge Avenue #3, Charleston, South Carolina, 29403, USA
Received: September 23, 2019; Accepted: November 18, 2019; Published: November 25, 2019
Abstract
Objective: To quantify and compare the severity of hearing loss in pediatric patients with various etiologies of hypothyroidism.
Study design: Retrospective review.
Setting: Tertiary referral hospital.
Patients: Children in the AudGen database with a diagnosis of congenital hypothyroidism, maternal hypothyroidism, iatrogenic hypothyroidism, iodine hypothyroidism, and Hashimoto hypothyroidism.
Interventions: None.
Main outcome measures: PTA4 values reflective of severity of hearing loss, change in PTA4 values over time representative of progression of hearing loss.
Results: Patients with congenital hypothyroidism had greater hearing loss than those with acquired (35.1 vs. 29.4, p=0.004). Patients with goitrous congenital hypothyroidism had greater hearing loss than those with nongoitrous (50.8 vs. 34.5, p=0.0370). Patients with Hashimoto thyroiditis demonstrated a vastly greater improvement rate in HL than patients with other types of hypothyroidism.
Conclusions: Initial hearing loss in patients with pediatric hypothyroidism is significantly greater in severity in patients with congenital as opposed to acquired causes of hypothyroidism. In patients with congenital hypothyroidism, severity of initial hearing loss is significantly greater in patients with a coexisting goiter compared to patients that lack a goiter. Improvements over time do not vary significantly by congenital vs. acquired and goitrous vs. nongoitrous etiologies of pediatric hypothyroidism.
Keywords: Pediatric hypothyroidism; Hearing loss.
Introduction
Congenital hypothyroidism occurs at an incidence of 1:2000 births [1]. There are various causes of hypothyroidism in children, including pre- and postnatal etiologies. Prenatal causes include iodine deficiency in utero, thyroid agenesis or aplasia, maternal hypothyroidism, and genetic defects in hormonogenesis [2]. Postnatal causes of hypothyroidism in children include iatrogenic either postiodine ablation or postsurgical, and autoimmune thyroiditis [2].
Hearing loss can occur in hypothyroidism, afflicting 25% of acquired and 35-50% of congenital hypothyroidism cases [3]. Because thyroid hormone is necessary during the critical period for ear development in utero, hypothyroidism occurring during this time can have lasting effects on hearing [4]. The cochlea is developed by week 15, and connections to the temporal lobe form during weeks 25-30 [5]. However, development of the ear structure continues throughout the first several years of life with reshaping of the vestibular duct occurring until 4 years of age [6]. Preceding ear structure development, the fetal thyroid develops by week 10-12, but does not start to produce thyroid hormone until 16-20 weeks. This means that transplacental maternal thyroid hormone is necessary until that time [5].
While the necessity of thyroid hormone for both the development and maintenance of auditory structures has been demonstrated by the presence of hearing loss in patients with both congenital and acquired hypothyroidism [4], differences in severity of hearing loss in these two populations has not been established. Furthermore, the progression of hearing loss in children with these conditions has not been studied extensively.
The AudGenDB, Audiologic and Genetic Database (AudGenDB), is an NIH funded [7], resource populated by 175,000 patients from the Children’s Hospital of Philadelphia. Data entries include patient demographics, diagnoses, procedures, radiology studies, audiograms, tympanograms, and Optoacoustic Emissions (OAE) test results. This database has been used in the past to describe the association between hearing loss and several medical conditions [7-9]. The purpose of this project was to investigate characteristics and progression of hearing loss in children with congenital and acquired hypothyroidism and determine changes over time for patients with both etiologies using the AudGenDB. We hypothesized that both groups of subjects would show greater prevalence of hearing loss than the general population, but that hearing loss would be more severe in those with congenital hypothyroidism.
Methods
Subjects
This study was exempt from the institutional review board review as all data was in the public domain. All data was obtained from the AudGenDB, which has been used to analyze the association with hearing loss and other medical conditions previously (insert 3 references). The following International Classification of Disease-9 (ICD-9) diagnoses were used to filter out study subjects: congenital hypothyroidism (243), goiter (240), maternal hypothyroidism (648.13), iodine hypothyroidism (244.2), Hashimoto thyroiditis (245.2), and iatrogenic hypothyroidism (244.1).
Subjects with these diagnoses were categorized into congenital and acquired groups, with the diagnosis of “maternal hypothyroidism” considered under the “congenital hypothyroidism” exposure category. Subjects with the diagnosis of “congenital hypothyroidism” were also sorted by presence of goiter, and patients with the diagnosis of “congenital hypothyroidism” and “mineral deficiency” were sorted an additional group (n=2). Subjects with acquired hypothyroidism were categorized by etiology: either iatrogenic, iodine, or Hashimoto thyroiditis. A flow diagram for patient categorization by diagnosis is displayed in (Figure 1).
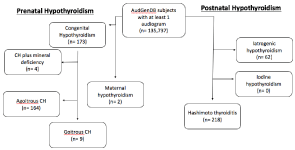
Figure 1: Flowchart for subject selection.
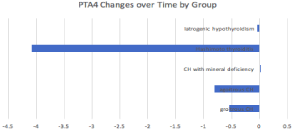
Figure 2: Mean change in PTA4 (in dB) from first to last audiogram over time
by type of pediatric hypothyroidism.
Audiologic evaluation
Pure tone average-4 (PTA4) values (mean of 500, 1000, 2000, and 4000 Hz data) were used to determine hearing outcomes for all subjects. As a baseline, we selected the first PTA4 indicative of hearing loss that was closest to the time of diagnosis of hypothyroidism. This was thought to be most representative of the peak severity of the hypothyroidism manifestations in these patients.
PTA4 changes over time were used to determine the trends for improvement or worsening of hearing loss in subjects. The changes over time were calculated as the difference in PTA4 values between the first audiologic testing appointment and the most recent appointment. Slope of PTA4 changes was calculated as change in PTA4 from first to last measurement divided by time interval between these two measurements (in years).
Statistical analysis
Independent samples t-test results were used to compare the mean PTA4 values, reflecting severity of subject hearing loss between congenital vs. acquired hypothyroidism at the time closest to diagnosis of hypothyroidism. A p-value of ‹0.05 was considered statistically significant. This same independent samples t-test analysis method was also used to compare hearing loss severity in goitrous vs. nongoitrous hypothyroidism. An analysis of variance was utilized to assess for differences in demographic information, including age, sex, race, and ethnicity of the various groups.
Results
Patients and demographics
There were a total of 459 children included in the study. These patients had the following diagnoses: congenital hypothyroidism with goiter (n=9), congenital hypothyroidism without goiter (n=164), congenital hypothyroidism with mineral deficiency (n=4), maternal hypothyroidism (n=2), Hashimoto thyroiditis (n=218), and iatrogenic hypothyroidism (n=62).
Patient demographics are presented in (Table 1). The congenital hypothyroidism with goiter group included 2 males and 7 females, of which 8 were white and 1 was coded as “other” for race. The congenital hypothyroidism without goiter group included 84 males and 81 females, of which 114 were white, 25 were black, 7 were Asian, and 19 were other. The congenital hypothyroidism with mineral deficiency cohort included 2 males and 2 females, of which 4 were white. The maternal hypothyroidism cohort consisted of 1 male and 1 female, both of which were white.
Diagnosis
N
Sex
Race
Ethnicity: Hispanic or latino?
Age of 1st audiogram in years
(mean & SD)
Time between 1st & last PTA4 in years
(mean & SD)
Congenital
Congenital hypothyroidism with goiter
9 (2.0%)
2 M
7 F
8 white
0 black
0 asian
1 other
0 yes
6 no
3 unknown
11.9 (8.9)
2.6 (2.4)
Congenital hypothyroidism without goiter
164
(35.7%)
84 M
81 F
114 white
25 black
7 asian
19 other
13 yes
144 no
13 unknown
7.1 (4.4)
3.3 (2.8)
Congenital hypothyroidism with mineral deficiency
4
(0.9%)
2 M
2 F
4 white
0 black
0 asian
0 other
1 yes
3 no
0 unknown
4.9 (0.9)
0.2
Maternal hypothyroidism
2
(0.4%)
1 M
1 F
2 white
0 black
0 asian
0 other
0 yes
2 no
0 unknown
9.5 (8.6)
2.2
All congenital
179
(39.0%)
89 M
91 F
128 white
25 black
7 asian
20 other
14 yes
155 no
16 unknown
7.4 (4.8)
3.3 (2.8)
Acquired
Hashimoto thyroiditis
218 (47.5%)
82 M
136 F
178 white
10 black
7 asian
23 other
17 yes
193 no
8 unknown
9.9 (5.4)
3.8 (3.5)
Iatrogenic hypothyroidism
62 (13.5%)
30 M
32 F
47 white
6 black
1 asian
8 other
8 yes
41 no
13 unknown
10.5 (5.7)
4.5 (3.1)
All acquired
280 (61.0%)
112 M
168 F
225 white
16 black
8 asian
31 other
25 yes
234 no
21 unknown
10.0 (5.5)
4.0 (3.4)
All patients
459 (100%)
201 M
259 F
353 white
41 black
15 asian
51 other
39 yes
389 no
37 unknown
9.0 (5.4)
3.7 (3.2)
Table 1: Subject demographics by group.
Age of first PTA4 in years for each cohort was as follows: 11.9 (congenital hypothyroidism without goiter), 7.1 (congenital hypothyroidism with goiter), 4.9 (congenital hypothyroidism with mineral deficiency), 9.5 (maternal hypothyroidism), 9.9 (Hashimoto thyroiditis), 10.5 (iatrogenic hypothyroidism). Analysis of variance was used to calculate for any significant difference between age of first PTA4 value between the congenital (mean age 7.4, standard deviation 4.8) and acquired (mean age 9.0, standard deviation 5.4) groups and was non-significant.
Of the entire congenital hypothyroidism cohort, 89 were male and 91 were female. 128 of these patients were white, of which 14 were Hispanic ethnicity. 25 patients were black, 7 were Asian, and 20 were classified as other race. The entire acquired hypothyroidism cohort included 201 males and 259 females. 353 of these patients were white, of which 39 were Hispanic ethnicity. 41 patients were black, 15 were Asian, and 51 were classified as other race. Comparison by analysis of variance of group demographics including sex, race, and ethnicity did not yield any significant difference between congenital vs. acquired hypothyroidism and goitrous vs. nongoitrous congenital hypothyroidism.
Severity of hearing loss
Mean first PTA4 indicating hearing loss for the entire cohort of patients with either congenital or acquired hypothyroidism was 31.63 (standard deviation 19.11). First PTA4 in dB for each cohort was as follows: 50.8 (congenital hypothyroidism without goiter), 34.5 (congenital hypothyroidism with goiter), 20.3 (congenital hypothyroidism with mineral deficiency), 41.9 (maternal hypothyroidism), 28.5 (Hashimoto thyroiditis), 32.5 (iatrogenic hypothyroidism).
Patients with congenital hypothyroidism had greater hearing loss than those with acquired hypothyroidism (PTA4 of 35.1 vs. 29.4, p=0.004).
Patients with goitrous congenital hypothyroidism had greater hearing loss than those with nongoitrous congenital hypothyroidism (PTA 4 of 50.8 vs. 34.5, p=0.0370).
Progression of hearing loss
Mean last PTA4 in dB for each cohort was as follows: 37.6 (congenital hypothyroidism without goiter), 33.6 (congenital hypothyroidism with goiter), 25.0 (congenital hypothyroidism with mineral deficiency), 22.8 (maternal hypothyroidism), 25.2 (Hashimoto thyroiditis), 32.5 (iatrogenic hypothyroidism). Mean changes in PTA4 from first to last PTA4 were calculated, and can be seen in (Table 2). Mean change from first to last PTA4 for each cohort was as follows: -0.5 (congenital hypothyroidism without goiter), -0.8 (congenital hypothyroidism with goiter), 0.02 (congenital hypothyroidism with mineral deficiency), -4.08 (Hashimoto thyroiditis), and -0.04 (iatrogenic hypothyroidism).
Diagnosis
N first
First PTA (mean & SD)
Last PTA
(mean & SD)
Change in PTA
Slope of change in PTA per year
Congenital
Congenital hypothyroidism
with goiter
9
(2.0%)
50.8 dB (8.9)
37.6 (25.5)
N=7
-0.5 dB (7.9)
-0.24 dB
Congenital hypothyroidism
without goiter
164 (35.7%)
34.5 dB (22.0)
33.6 (23.3) N=124
-0.8 dB (11.8)
0.53 dB
Congenital hypothyroidism with mineral deficiency
4
(0.9%)
20.3 dB
(3.6)
25.0
N=1
0.02 dB
(0)
-0.01 dB
Maternal hypothyroidism
2 (0.4%)
41.9 dB (5.3)
22.8 (11.8)
N=2
No follow-up
N/A
All congenital
179
(39.0%)
35.1 dB (22.6)
33.5
(23.13) N=135
-1.01 (11.79) N=135
0.36 dB
Acquired
Hashimoto thyroiditis
218 (47.5%)
28.5 dB (14.8)
25.2 (17.38) N=178
-4.1 dB (11.1)
-5.57 dB
Iatrogenic hypothyroidism
62 (13.5%)
32.5 dB (20.06)
32.5 (21.65)
N= 49
-0.04 dB (11.1)
0.08 dB
All acquired
280 (61.0%)
29.4 dB (16.18)
26.8
(18.62) N=227
-3.2 (11.25) N=227
-4.35
All patients
459 (100%)
31.6 (19.11)
29.0 (20.41) N=362
-2.4 (11.49) N=362
0.64 dB
Table 2: PTA4 values and changes with time by diagnosis.
Patients with Hashimoto thyroiditis demonstrated a vastly greater improvement rate in HL than patients with other types of hypothyroidism, depicted in (Figure 1). Of note, the maternal hypothyroidism and iodine hypothyroidism groups did not have any patients with follow-up audiograms performed so these groups were not included in (Figure 1). Slope of PTA4 change per year is also displayed in (Table 2). Slope of PTA4 in dB/year for each cohort was as follows: -0.24 (congenital hypothyroidism without goiter), 0.53 (congenital hypothyroidism with goiter), -0.01 (congenital hypothyroidism with mineral deficiency), -5.57 (Hashimoto thyroiditis), and 0.08 (iatrogenic hypothyroidism).
Patients with congenital hypothyroidism had greater hearing loss progression over time than those with acquired (0.36 vs. -4.35 dB/ year, p=0.034). Patients with goitrous congenital hypothyroidism had less hearing loss progression over time than those with nongoitrous congenital hypothyroidism (-0.24 vs. 0.53 dB/year, p=0.926).
Discussion
Our results indicate that patients with congenital and acquired hypothyroidism demonstrated PTA values in the hearing loss range. However, mean PTA was significantly higher in the group of patients with congenital hypothyroidism. This can likely be explained by differences in the mechanism of the associated hearing losses, and the time of incidence of these conditions in relation to the critical period of audiologic development. Essentially, cases of acquired hypothyroidism-associated hearing loss occur solely as a consequence of lack of thyroid hormone supply, while congenital hypothyroidism includes the same mechanism at a time when T3 and T4 are necessary for permanent developments in middle and inner ear and central nervous system structure [10].
Causes of acquired hypothyroidism in children include autoimmune thyroiditis, postviral thyroiditis, suppurative thyroiditis secondary to bacterial infection, acquired iodine deficiency, medication side effects, and iatrogenic causes including radioactive ablation and thyroidectomy [11]. Thyroid hormones serve as transcription factors in the regulation of expression of prestin, a motor protein in cochlear hair cells5. Additionally, thyroid hormone deficiency in patients with acquired hypothyroidism results in a decrease in cell energy, and subsequent decreases in rates of inner ear organ oxygenation and protein production for myelin in the central nervous system [3]. Low serum thyroid hormone levels cause low expression of KCNQ4 potassium channels in the cochlea, resulting in decreased endolymphatic potential and low cochlear amplification [10]. Another cause of sensorineural hearing loss is cochlear damage caused by mucopolysaccharide accumulation in the cochlea and tectorial membrane [2]. Conductive hearing loss in patients with acquired hypothyroidism is a result of edema and hypertrophy of both the eustachian tube and middle ear, leading to decreased compliance in these structures and in the formation of myxedematous middle ear effusion [2]. Reversibility of hearing loss in patients with acquired hypothyroidism is unclear, but is less likely to be reversible with increased duration from incidence to initiation of treatment [12].
Primary congenital hypothyroidism refers to any condition with low levels of serum thyroid hormone and increased Serum Thyrotropin (TSH) that present at birth [13]. This is in contrast to central hypothyroidism, which refers to hypothyroidism occurring as a result of decreased serum TSH due to failure of either the hypothalamus or anterior pituitary [12]. The incidence of congenital hypothyroidism approximates 1:2000 births [11], with hearing disorders occurring 100 times more frequently in pediatric patients with congenital hypothyroidism than in euthyroid patients [10]. The most common causes of congenital hypothyroidism include thyroid gland dysgenesis and dyshormonogenesis [11]. Dysgenic or embryogenic causes, including agenesis, hypoplasia, and ectopy [14], account for approximately 80% of congenital hypothyroidism cases [11,13,15], while 15-20% of cases of congenital hypothyroidism are caused by hormone synthesis defects. Common dyshormonogenic mutations include those in TPO, NIS, TG, DUOX2, and SLC26A4 genes [14]. SLC26A4 mutations fall under the domain of Pendred syndrome, which produces a triad of sensorineural hearing loss, goiter formation, and either hypothyroidism or euthyroidism [5]. Most patients with Pendred syndrome also exhibit enlarged vestibular aqueductal structures on imaging in addition to sensorineural hearing loss, encompassing a condition called Mondini syndrome [6]. This is hypothesized to occur as a result of insult from low serum thyroid hormone levels early in development that causes cochlear dysplasia [6].
In addition to dysgenesis and dyshormonogenesis, other known causes of antenatal hypothyroidism include maternal ingestion of antithyroid drugs, maternal antibodies blocking the fetal TSH receptors, and either iodine deficiency or excess [11], in which case too much iodine causes suspension of thyroid hormone synthesis per the Wolff-Chaikoff effect [16,17].
It is generally understood that congenital hypothyroidism results in bilateral symmetrical hearing loss that is more frequently sensorineural than conductive [4]. Thyroid hormone, especially T3, is important for inner ear development. A deficiency in this hormone causes impaired epithelial maturation in the cochlea [4] and permanent repression of cochlear potassium channels necessary for propagating an auditory signal [18]. The outer hair cells in the tectorial membrane, along with the central nervous system axonal myelination and maturation process are also underdeveloped if not exposed to adequate thyroid hormone during the critical development period [10]. Any conductive hearing loss is thought to result from hypothyroidism-induced middle ear defects [4], specifically permanent alterations in the ossicular structures [5]. The best predictor of permanent hearing damage in rats undergoing fetal development in a hypothyroidism simulation has been found to be postnatal serum T4 [19]. The prognosis for reversibility of congenital hypothyroidism-induced hearing loss is not certain, and it has been estimated that hearing loss persists in approximately 25% of pediatric patients who have undergone early treatment with levothyroxine [20]. Later initiation of levothyroxine treatment is associated with an increased risk chance for permanent hearing loss, and it is recommended that treatment should be initiated by the 3rd week of life [5]. Furthermore, later treatment initiation can be associated with reduced scores in language and speech comprehension, which improve with the implementation of a hearing aid [5]. One important consideration for prenatally detectable congenital hypothyroidism is the possibility for intraamniotic treatment with levothyroxine. This has been proven to improve the neuro-auditory effects, specifically, of deprivation of thyroid hormone levels during intrauterine development [17]. Our results indicate that, in patients with congenital causes of pediatric hypothyroidism, change in PTA4 per year is minimal (-1.01 dB/year). This means that the baseline or initial PTA4 value obtained from the patient’s first audiogram has far greater prognostic value on the patient’s future hearing status than does the possibility for improvement over time.
Our study also found a significant increase in PTA values in pediatric patients with congenital hypothyroidism and goiter, compared to agoitrous patients with congenital hypothyroidism. In 1988, the Myxedematous Committee of the Clinical Society of London acknowledged that there was a known association between the presence of a goiter and hearing loss [21]. It is possible that there is an association with severity of hearing loss and presence of a goiter due to the fact that etiologies responsible for the formation of a goiter occur at the cell membrane level, and that these same changes are occuring in the cell membrane of the cochlea. This would mean that deficiency of thyroid hormone is only part of the etiology, and that this deficiency synergizes with other etiologies. One important pathology that reflects this possibility is Pendred syndrome, which occur as a result of mutations in the SLC26A4 gene that codes for pendrin. Pendrin is an iodine transporter that exists in both the thyroid follicular cells and the inner ear [5], so mutations in the transporter existing on the inner ear alone would explain hearing loss either independently of or synergistically with a deficiency in thyroid hormone supply. While presence of goiter, hearing loss, and thyroid pathology are the hallmark of Pendred syndrome, the patient can sometimes be euthyroid so it is important to consider that not all patients with Pendred syndrome have been captured by the “congenital hypothyroidism” diagnosis via the ICD-9 code in AugGen. Additionally, Pendred syndrome is only one of many possible causes of neonatal goiter formation. Other common causes include iodine excess or deficiency, the presence of maternal antibodies, excess maternal use of thionamine medication, and other congenital hormonal imbalances [17]. Known consequences of fetal goiter include compression of the trachea with subsequent asphyxia, esophageal compression with subsequent polyhydramnios, arteriovenous shunt with subsequent high output heart failure, preterm delivery risk, and shoulder dystocia due to neck hyperextension during delivery [17]. It is important to consider the fact that these potential consequences can have long term sequelae that could secondarily impact fetal hearing function as well. It is notable that, in this study, the changes in PTA4 per year were not significantly different in the goitrous vs. agoitrous groups of patients with congenital hypothyroidism. This emphasizes the importance of the baseline audiogram as the major indicator of future hearing prognosis for the patient.
Limitations for this study are mostly related to limitations with the use of the AudGen database, which sorts patients by ICD-9 diagnostic code and does not include newborn screening. This limits the amount of diagnostic criteria that can be assessed in the study. For example, iodine deficiency is not its own ICD-9 code, but falls under the code for mineral deficiency. Furthermore, patients with the ICD- 9 code for congenital hypothyroidism can be sorted into categories such as “goiter” or “no goiter,” but the etiology of the congenital hypothyroidism is not always clear. The first PTA’s for each patient used for this study are the first PTA values found on record in the AudGen database, but these PTA values were not all collected at the same age for each subject. Additionally the mean of the PTA values was used which averaged the left and right ears tests when both tests were available. Similarly, treatment status for each of these patients, and severity of the original congenital hypothyroidism is unknown. Since AudGen database reflects a population of patients receiving auditory testing, it is a considerable possibility that hearing loss is overly represented in this population.
Conclusion
Initial hearing loss in patients with pediatric hypothyroidism is significantly greater in severity in patients with congenital as opposed to acquired causes of hypothyroidism. In patients with congenital hypothyroidism, severity of initial hearing loss is significantly greater in patients with a coexisting goiter compared to patients that lack a goiter. Improvements over time do not vary significantly by congenital vs. acquired and goitrous vs. nongoitrous etiologies of pediatric hypothyroidism. Prompt diagnosis and treatment of patients with hypothyroidism-associated hearing loss is vital to support proper social and intellectual learning in the developing pediatric patient.
References
- Christine EC, Wassner AJ. Congenital hypothyroidism: insights into pathogenesis and treatment. Int J Pediatr Endocrinol. 2017; 11.
- Santos K, Dias N, Mazeto G, Carvalho L, Lapate RL, Martins R. Audiologic evaluation in patients with acquired hypothyroidism. Braz J Otorhinolaryngol. 2010; 76: 478-484.
- Lichtenberger GL, Santos DS, Hassani Y, Ecosse E, Van DAT, Léger J. Factors Associated With Hearing Impairment in Patients With Congenital Hypothyroidism Treated Since the Neonatal Period: A National Population- Based Study. The Journal of Clinical Endocrinology & Metabolism. 2013; 98: 3644-3652.
- Melse BA, Mackenzie I. Iodine deficiency, thyroid function and hearing deficit: a review. Nutr Res Rev. 2013; 26: 110-117.
- Callison DM, Karl LH. Large Vestibular Aqueduct Syndrome: An Overlooked Etiology for Progressive Childhood Hearing Loss. J Am Acad Audiol. 1998; 9: 285-291.
- Andrade C, Machado GC, Fernandes L, Albuquerque JM, Silva LC, Romos HS, et al. Mechanisms involved in hearing disorders of thyroid ontogeny: a literature review. Arch Endocrinol Metab. 2017; 61: 501-505.
- Muus JS, Weir FW, Kreicher KL, Bowlby DA, Discolo CM, Meyer TA. Hearing loss in children with growth hormone deficiency. Int J Pediatr Otorhinolaryngol. 2017; 100: 107-113.
- Kreicher KL, Schopper HK, Naik AN, Hatch JL, Meyer TA. Hearing loss in children with primary ciliary dyskinesia. Int J Pediatr Otorhinolaryngol. 2018; 104: 161-165.
- Weir FW, Hatch JL, McRackan TR, Wallace SA, Meyer TA. Hearing Loss in Pediatric Patients With Cerebral Palsy. Otology & Neurotology. 2018; 39: 59-64.
- Wassner AJ. Pediatric Hypothyroidism: Diagnosis and Treatment. Pediatric Drugs. 2017; 19: 291-301.
- Malik V, Shukla GK, Bhatia N. Hearing Profile in Hypothyroidism. Indian Journal of Otolaryngology and Head and Neck Surgery. 2002; 54: 285-290.
- Wasniewska M, Filippo DL, Siclari S, Salzano G, Messina MF, Lombardo F, et al. Hearing loss in congenital hypothalamic hypothyroidism: a wide therapeutic window. Hear Res. 2002; 172: 87-91.
- Macchia PE. Recent advances in understanding the molecular basis of primary congenital hypothyroidism. Mol Med Today. 2000; 6: 36-42.
- Kuhnen P, Turan S, Frohler S, Guran T, Aali S, Beibermann H, et al. Identification of PENDRIN (SLC26A4) Mutations in Patients With Congenital Hypothyroidism and “Apparent” Thyroid Dysgenesis. The Journal of Clinical Endocrinology & Metabolism. 2014; 99: 169-176.
- Pfarr N, Borck G, Turk A, Napiontek U, Keilmann A, Wibke MF, et al. Goitrous Congenital Hypothyroidism and Hearing Impairment Associated with Mutations in theTPOandSLC26A4/PDSGenes. The Journal of Clinical Endocrinology & Metabolism. 2006; 91: 2678-2681.
- Overcash RT, Marc-Aurele KL, Hull AD, Ramos GA. Maternal Iodine Exposure: A Case of Fetal Goiter and Neonatal Hearing Loss. Pediatrics. 2016; 137:e20153722-e20153722.
- Hardley MT, Chon AH, Mestman J, Nguyen CT, Geffner ME, Chmait RH. Iodine-Induced Fetal Hypothyroidism: Diagnosis and Treatment with Intra- Amniotic Levothyroxine. Horm Res Paediatr. 2018; 90: 419-423.
- Song L, McGee J, Walsh EJ. The Influence of Thyroid Hormone Deficiency on the Development of Cochlear Nonlinearities. Journal of the Association for Research in Otolaryngology. 2008; 9: 464-476.
- Crofton K. Developmental Disruption of Thyroid Hormone: Correlations with Hearing Dysfunction in Rats. Risk Analysis. 2004; 24: 1665-1671.
- Bruno R, Aversa T, Catena M, Valenzise M, Lombardo F, Filippo DL, et al. Even in the era of congenital hypothyroidism screening mild and subclinical sensorineural hearing loss remains a relatively common complication of severe congenital hypothyroidism. Hear Res. 2015; 327: 43-47.
- Comer DM, McConnell EM. Hypothyroid-associated sensorineuronal deafness. Ir J Med Sci. 2010; 179: 621-622.