
Case Report
J Pediatri Endocrinol. 2022; 7(1): 1052.
Growth Failure due to JAK Inhibition Overcome by Recombinant IGF-I
Hutchison MR*
Department of Pediatrics, University of Arkansas for Medical Sciences, USA
*Corresponding author: Hutchison MR, Department of Pediatrics, University of Arkansas for Medical Sciences, USA
Received: June 22, 2022; Accepted: July 22, 2022; Published: July 29, 2022
Abstract
Introduction: Normal linear growth requires an intact GH/IGF-I axis, wherein GH from the pituitary induces the synthesis and release of IGF-I from the hepatocyte followed by IGF-I-mediated chondrocyte division at the growth plate. At the hepatocyte, GH binding to its cognate receptor results in a conformational change in the intracellular portion of the receptor that recruits and activates the tyrosine kinase JAK2. JAK2 then phosphorylates the transcription factor Stat5b which allows it to bind to the IGF-I promotor and increase IGF-I production. JAK kinase inhibitors are used for the treatment of inflammatory disorders such as rheumatoid arthritis, and theoretically would be expected to interfere with linear growth by impairing IGF-I production. We present a case of growth failure due to JAK2 inhibition and successful treatment with recombinant IGF-I therapy.
Case Presentation: An 8-year-old Caucasian male presented to our endocrine clinic with a complaint of poor linear growth. He has been followed at NIH since infancy for a diagnosis of Chronic Atypical Neutrophilic Dermatosis with Lipodystrophy and Elevated temperature (CANDLE)-like syndrome. He was placed on oral prednisone at 6 months of age. At 14 months of age he started the Jak1/Jak2 inhibitor baricitnib, after which the prednisone dose was gradually decreased. His symptoms improved after starting the JAK inhibitor. However, his linear growth was poor; height SDS went from -2.2 at 3 years to -4.2 at 8 years. When seen initially in our clinic, his IGF-I was very low at 17ng/ ml. After recombinant human IGF-I daily injections were started his linear growth velocity improved from 2.5 to 9.8 cm/year, and his height SDS improved to -2.0 by age 10.
Conclusions: The use of JAK inhibitors for inflammatory disorders can result in growth failure in children, and can be overcome with rhIGF-I.
Keywords: Growth; IGF-I; JAK kinase
Introduction
Linear growth in a healthy child requires an intact GH/IGF-I axis, wherein GH from the pituitary induces the synthesis and release of IGF-I from the hepatocyte. IGF-I circulates bound to specific binding proteins, and at the growth plate is released from the binding protein todirectly stimulate chondrocyte division [1]. At the hepatocyte, GH binding to its cognate receptor results in a conformational change in the intracellular portion of the receptor that recruits the tyrosine kinase JAK2, allowing activation of the kinase via autophosphorylation. JAK2 then phosphorylates the GH receptor, which leads to recruitment and activation of STATs (Signal Transducer and Activator of Transcription). TheSTAT5btranscription factor then binds to the IGF-I promotor and increases IGF-I production [2].
The JAK family of tyrosine kinases also play a role in cytokine signaling [3]. Like the GH receptor, cytokine receptors lack catalytic kinase activity and rely on the JAK family of kinases to transmit intracellular signals via phosphorylation of downstream STAT proteins [4]. JAK inhibitors such as baricitinib are approved by the FDA for the treatment of inflammatory diseases such as rheumatoid arthritis not controlled by TNF inhibitors [5]. The use of JAK inhibitors in growing children raises the possibility of growth suppression. We present a case of a child who presented with growth failure while taking a JAK inhibitor and his improved growth when treated with recombinant human IGF-I.
Case Presentation
An 8-year-old male presented to our endocrinology clinic for evaluation of poor linear growth. He was born at 40 weeks after an uncomplicated pregnancy and had no perinatal complications. On day of life two he developed a fine popular rash that quickly became a generalized popular/macular rash. Over the next few weeks he developed intermitted swelling of the fingers, toes, and dorsum of the feet, followed by new symptoms of intermittent fevers to a max of 101.7 and persistent lymphopenia. He was evaluated at NIH and given a diagnosis of a CANDLE-like disorder. Subsequent genetic testing revealed a heterozygous frame-shift mutation in the SAMD9L gene. At 6 months of age he started glucocorticoid treatment at a dose of 14 mg/m2/day hydrocortisone equivalent. His parents reported that the steroids did not seem to alter his symptoms. At 13 months he was found to be hypertensive, and started an ACE inhibitor at 1 mg/ day. He was diagnosed with failure to thrive due to poor po intake; a gastrostomy tube was placed at age 3 years, and then removed at 7 years of age when his oral intake improved.
Because the glucocorticoids were not having any effect on his disease progression, at the age of 14 months he was started on the JAK inhibitor baricitinib on a compassionate care basis. The initial dose was 2 mg 4 times daily. His parents reported a positive response to the medication, with fewer episodes of fever, pain, and rash. Over the ensuing months the steroid dose was gradually decreased, and then stopped altogether at age 6 years.
At 4 years of age the lymphopenia worsened; bone marrow biopsies ruled out dysplasia and malignancy. The problem was thought to be due to the JAK inhibitor, and the dose was reduced to 2 mg 3 times daily. His parents noted an increase in his symptoms after the decreased dose. He also developed positive BK viral positivity at age 3 years, which was also attributed to the JAK inhibitor.
When he was initially seen in the Endocrinology clinic his height SD score was quite low at -4.2 below the mean for age. The initial work up for short stature revealed a very low serum IGF-I level of 17 ng/ml. GH stimulation testing with two stimuli (arginine and insulin) resulted in a peak serum GH of 15.2ng/ml, ruling out GH deficiency as a cause of the poor growth. Recombinant human IGF-I was started at a dose of 80 mcg/kg twice daily and quickly increased to the target dose of 120 mcg/kg twice daily without any hypoglycemic symptoms. His growth velocity prior to the rhIGF-I was poor at 2.5 cm/yr, but increased to almost 10 cm/yr on rhIGF-I therapy. His height SDS improved from -4.2 at 8 years of age to -2.0 at age 10. Per the family the rhIGF-I have been well-tolerated, and has not had any negative impact on the course of his primary disease.
Discussion
The JAK family of kinases are critical to cytokine signaling and thus make an ideal drug target for certain inflammatory conditions. The GH receptor belongs to the same receptor class as the cytokine receptors, and thus signals via the JAK/STAT pathway as well. Germline mutations in the transcription factor STAT5b cause short stature with GH resistance [6]. Germline mutations in JAK2 would also presumably cause short stature, but the reported phenotype of these patients is a high risk for certain hematopoetic malignancies [7]. Because JAK2 is needed for GH signaling, it stands to reason that inhibition of this kinase would result in GH resistance and poor growth.
Our patient had been receiving the JAK inhibitor baricitinib for almost 7 years at the time he presented with significant growth failure. He continued to show poor linear growth after discontinuation of glucocorticoid medication. The extremely low IGF-I level but normal response to the GH stimulation test is consistent with a state of GH resistance. His family was eager to find a therapy that would improve his growth, so we elected to user hIGF-I to bypass the inhibition of the JAK2 kinase. His response to the therapy was robust, and after 2 years on this treatment he continues to show an improved growth rate, despite the re-initiation of glucocorticoids at age 9.5 years. Moreover, there has been no worsening of his primary disease process due to the rhIGF-I.
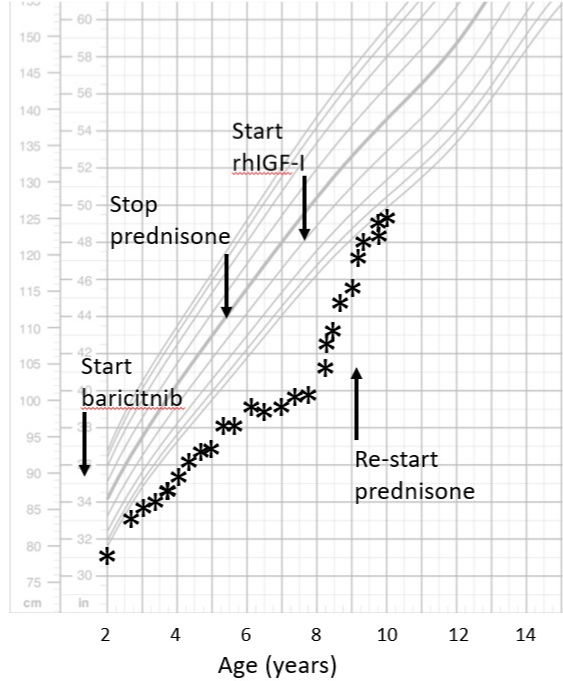
Figure 1: Growth Chart.
Conclusions
We present a case of growth failure in an 8-year-old male due to inhibition of the JAK tyrosine kinase that is needed for GH regulation of IGF-I synthesis. This case underscores the potential for growth failure in children treated with JAK inhibitors, and the potential reversal of the poor growth with rhIGF-I therapy.
References
- Isaksson O. GH, IGF-I, and growth. Journal of pediatric endocrinology & metabolism: JPEM. 2004; 17: 1321-6.
- Rotwein P. Mapping the growth hormone–Stat5b–IGF-I transcriptional circuit. Trends in Endocrinology & Metabolism. 2012; 23: 186-193.
- Morris R, Kershaw NJ, Babon JJ. The molecular detaiols of cytokine signaling via the JAK/STAT pathway. Protein Sci. 2018; 27: 1984-2009.
- Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017; 17: 78.
- Hwa V, Nadeau K, Wit JM, Rosenfeld RG. STAT5b deficiency: lessons from STAT5b gene mutations. Best practice & research. Clinical endocrinology & metabolism. 2011; 25: 61-75.
- Goldin LR, Björkholm M, Kristinsson SY, Samuelsson J, Landgren O. Germline and somatic JAK2 mutations and susceptibility to chronic myeloproliferative neoplasms. Genome Medicine. 2009; 1: 55-55.