
Case Report
J Pediatri Endocrinol. 2022; 7(1): 1053.
Skeletal Abnormalities in a Patient with Trisomy 21 and ABCD Syndrome
Crawford B1,2*, Hutchison M1,2, Rowell A1,3 and Devore C1
¹Department of Pediatric Nephrology, Arkansas Children’s Hospital, 1 Children’s Hospital, USA
²Department of Pediatrics, University of Arkansas Medical Science, USA
³Department of Radiology, University of Arkansas Medical Science, USA
*Corresponding author: Brendan Crawford, Arkansas Children’s Hospital, Children’s Hospital, Little Rock AR 72202, USA
Received: July 11, 2022; Accepted: August 08, 2022; Published: August 15, 2022
Abstract
We report a 2-year-old girl presenting for failure to thrive noted to have hypercalcemia, renal dysfunction, and nephrocalcinosis. Initial skeletal imaging revealed several abnormalities of bone mineralization, not previously reported in limited case reports. Significant dietary calcium restriction resulted in normal serum calcium levels but also improvement in skeletal abnormalities. Kidney function improved but remained abnormal several years after presentation.
Keywords: Trisomy 21; Hypercalcemia; ABCD syndrome
Introduction
Trisomy 21 has been associated with multiple medical conditions affecting numerous organs throughout the body. Health supervision guidelines exist to guide the clinician through specific screening throughout childhood focusing on common conditions such as cardiac, thyroid, or ophthalmologic disease [1]. Trisomy 21 has also been associated with a rare condition, ABCD syndrome (AB-normal Calcium, Creatinine, Calcinosis in Down syndrome), characterized by hypercalcemia with associated nephrocalcinosis and renal dysfunction. To date, eight case reports have been published although follow-up time remains limited and there has been no report of skeletal involvement [2-9]. In this study, we present the ninth report with unique laboratory and skeletal imaging findings and detail a 3-year follow-up course.
Case Presentation
We report a 2-year-old girl with Trisomy 21 underwent evaluation for weight gain. Review of Down syndrome growth chart showed progressive weight gain up until 12 months of life (-0.2 SD), but then weight loss by 20 months (-1.66 SD). Formula was adjusted to promote weight gain with Nutren Jr formula (Abbott Nutrition, Chicago, IL) advanced to 22 kcal/oz, receiving 35-40 ounces per day to receive 95% caloric need (136% adequate calcium intake, 155% recommended dietary intake for phosphorus).
Medical history was noteworthy for pregnancy complicated by gestational diabetes, low birth weight, brief post-natal hospitalization for respiratory disease. Congenital heart disease was repaired at 6 months with no subsequent issues. Genetic testing confirmed Trisomy 21 and subsequent whole exome sequencing revealed no other abnormalities. Family history was negative for kidney, endocrine or other findings. Review of systems noteworthy for constipation but good urine output. Medication history was negative for alkali, vitamin, or calcium supplementation.
Initial physical exam showed typical facies of Trisomy 21, wellhealed surgical scar from repaired congenital heart disease but no other abnormal findings. Screening labs showed mildly elevated calcium (11.2 mg/dl), mildly elevated phosphorus (6.9 mg/dl) and moderate renal dysfunction confirmed on repeated testing (creatinine 1.2 mg/dl). The 25-hydroxyvitamin D was normal at 46.2 ng/ml, 1, 25-hydroxyvitamin D was low at 19 pg/ml (range 20-79 pg/ ml), and Parathyroid Hormone (PTH) was low at 18 pg/ml (range 12-65 pg/ml). Spot urine studies on 3 occasions within a few weeks suggested mild hypercalciuria (calcium- creatinine ratio 0.8- 1.05 mg/ mg). Renal ultrasound demonstrated symmetric, appropriately sized kidneys with both cortical and medullary nephrocalcinosis. A skeletal survey revealed multiple long bones with wide, lucent bands at the metaphysis and a “bone within bone” appearance at the epiphyses, as well as soft tissue calcifications at the posterolateral left thigh without overlying skin changes or known trauma to the site (Figure 1A).
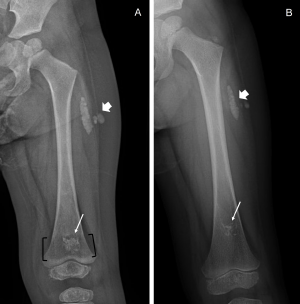
Figure 1A &B: (Image A) Left femur radiograph taken at time of initial
presentation. Wide, lucent metaphyseal band involving the distal femur
(black brackets) with central sclerosis (white arrow) creating a bone-inbone
appearance. Similar bone-in-bone appearance of the distal femoral
and proximal tibial epiphyses (not marked). Dense, lobular soft tissue
calcifications (white arrowhead) are located adjacent to the proximal femoral
shaft. (Image B) Left femur radiograph taken 1 year after the initial study.
Distal femur lucent metaphyseal band is no longer present, and the sclerotic
area has decreased in size. Proximal migration of the sclerotic bone is due
to internal growth at the distal femur. Soft tissue calcifications in the proximal
thigh (white arrowhead) have decreased in size.
Additional testing to identify the etiology of hypercalcemia revealed normal thyroid studies. Vitamin A level was mildly elevated at 0.95 mg/l (range 0.2-0.5 mg/l) and Parathyroid Hormone Related Peptide (PTHrP) was elevated at 9.1 pmol/L (normal below 0.2 pmol/l). PTHrP remained persistently elevated upon repeat testing. Hemoglobin, platelet, and lactate dehydrogenase levels were normal. CT and MRI whole body scans did not identify any solid organ malignancy.
Saline hydration and loop diuretic did not significantly alter calcium levels. Administration of calcitonin did transiently decrease serum calcium level from 11 mg/dl to 9 mg/dl, but levels rebounded shortly thereafter. Formula was changed from Nutren Jr to the calcium-deficient, vitamin D-free Calcilo XD (Abbott Nutrition, Chicago IL), which would provide only 3.5% recommended dietary intake. Following the formula change, serum calcium levels decreased over a period of weeks toward new baseline 9.8-10.5 mg/dl, an ageappropriate range. Similarly, renal dysfunction slowly improved over months to 0.8 mg/dl after several months, although still represents moderate renal dysfunction (estimated GFR 38 ml/min/1.73 m2 using modified Schwartz equation) [10].
The patient remained on calcium-deficient formula with slow introduction of low- calcium table foods with no recurrence of hypercalcemia. Repeat renal imaging after 6 months showed stable nephrocalcinosis. Repeat radiographs at 12 months (while still on low calcium formula) showed improved mineralization of the bones with improved areas of sclerosis (Figure 1B). After several months of calcium-deficient formula, patient developed mild hyperparathyroidism (204 pg/ml) without concomitant hypocalcemia or hyperphosphatemia, and PTHrP remained persistently elevated. After 2 years, formula was slowly transitioned to low-potassium lowphosphorusSuplena (Abbott Nutrition, Chicago IL) without resultant hypercalcemia or worsening renal dysfunction. Suplena regimen contains 43% adequate intake, which still represented a reduction from previous. On follow-up 3 years after initial evaluation, renal function remains moderately diminished (creatinine 0.8 mg/dl, estimated GFR 48 ml/min/1.73 m2 using modified Schwartz equation), classified as stage 3 chronic kidney diseases. The patient has continued to gain weight and progress in developmental milestones.
Discussion
Hypercalcemia can result from an extensive number of causes, although the exact mechanism in ABCD syndrome remains unclear. In the reported cases, parathyroid hormone (PTH) and 1, 25-OHD levels were suppressed in the setting of hypercalcemia. Almost all had a normal 25-hydroxyvitamin D level, whereas one toddler had only minimal elevated level [2-4,9]. In two toddlers, high consumption of cows’ milk was identified as a possible contributing factor [2- 5]. However, Cobenas and colleagues noted their patient did not consume significant cows’ milk, and instead increased intestinal calcium absorption by unknown mechanism was underlying the hypercalcemia [4]. Importantly, all children demonstrated improvement in hypercalcemia following calcium restricted diet, supporting the absorptive hypercalcemia hypothesis.
Two children in the published case reports had PTHrP checked, which was normal in one case [5]. In the other case, Nguyen and colleagues found markedly elevated PTHrP and subsequent diagnostic evaluation was undertaken although no cancerous etiology identified; the elevated PTHrP was thought to be secondary to renal insufficiency [7]. In our patient, the PTHrP levels remained markedly elevated for over six months. Our patient had low 1, 25-hydroxyvitamin D which has been seen in hypercalcemia of malignancy caused by elevated PTHrP levels [11]. PTHrP acts through the PTH receptor to drive calcium release from bone via osteoblast activation and increase phosphorus excretion in the renal tubule, but for unclear reasons PTHrP does not activate 1-a hydroxylase in the renal tubule (responsible for conversion of 25-vitaminhydroxy D to 1,25-vitaminhydroxy D). High PTHrP levels could explain the finding of hypercalcemia with normal phosphorus levels in the setting of low PTH in these patients with unexplained hypercalcemia. The C-terminus of PTHrP has been shown to accumulate in renal insufficiency whereas the N-terminus PTHrP should not accumulate [12]. It is unclear whether the elevated PTHrP in our patient represents artifact from delayed renal clearance versus a putative contributory factor in the underlying pathophysiology of ABCD syndrome.
While ABCD syndrome was first reported over 20 years ago, we report the first case with skeletal changes. The “bone within a bone” radiologicfinding can be seen in a wide variety of conditions, such as disordered bone growth or mineralization defects [13]. While low calcium state typically predisposes to mineralization abnormalities, reduced dietary calcium intake in our patient actually improved the skeletal changes. Ectopic tissue calcification has been reported in other conditions of hypercalcemia, although interestingly our patient only demonstrated mild hypercalcemia.
Conclusion
ABCD syndrome is a rare syndrome seen in toddlers with Trisomy 21consisting of hypercalcemia, renal dysfunction and nephrocalcinosis, often presenting with failure to thrive. Limited case reports have not identified exact mechanism of hypercalcemia hypercalcemia. Like other reported cases, dietary calcium restriction remains mainstay of treatment, supporting potential mechanism of increased intestinal absorption. The role of PTHrP to the pathogenesis remains unclear. Our case highlights potential for chronic kidney disease and skeletal changes.
Conflict of Interest
All authors report no financial disclosures or conflict of interest.
References
- Bull MJ, Committee on Genetics (2011) Health supervision for children with Down syndrome. Pediatrics. 2011; 128: 393-406.
- Proesmans W, Cock PD, Eyskens B. A toddler with Down syndrome, hypercalcaemia, hypercalciuria, medullary nephrocalcinosis and renal failure. Pediatric Nephrology. 1995; 9: 112-114.
- Thangaraju G, Hosduga S. G272(P) ABCD (AB-Normal Calcium, Calcinosis, Creatinine) Syndrome of Down Syndrome. Archives of Disease in Childhood. 2017; 102: A107-A107.
- Cobeñas C, Spizzirri F, Zanetta D. Another toddler with Down syndrome, nephrocalcinosis, hypercalcemia, and hypercalciuria. Pediatric nephrology. 1998; 12: 432.
- Ramage IJ, Durkan A, Walker K, Beattie TJ. Hypercalcaemia in association with trisomy 21 (Down’s syndrome). Journal of clinical pathology. 2002; 55: 543-544.
- Andreoli SP, Revkees S, Bull M. Hypercalcemia, hypercalciuria, medullary nephrocalcinosis, and renal insufficiency in a toddler with Down syndrome. Pediatric Nephrology. 1995; 9: 673-673.
- Nguyen M, Litra F, Kamil A, Ergun-Longmire B. Intractable Vomiting in an 11-Month-Old Boy With Trisomy 21: A Case Report on Abnormal Calcium/ Calcinosis/Creatinine in Down Syndrome. Cureus. 2021; 13.
- Tran HA, Song S, Crock PA, Mattes J, Howard K. The A, B, C, D of hypercalcaemia in Down syndrome. BMJ Case Reports. 2009; 2009: bcr0620080232-bcr0620080232.
- Filler G, Kotecha S, Milanska J, Lawson ML. Trisomy 21 with hypercalcemia, hypercalciuria, medullary calcinosis and renal failure--a syndrome?. Pediatric nephrology. 2001; 16: 99-100.
- Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. Journal of the American Society of Nephrology : JASN. 2009; 20: 629-637.
- Asonitis N, Angelousi A, Zafeiris C, Lambrou GI, Dontas I, Kassi E. Diagnosis, Pathophysiology and Management of Hypercalcemia in Malignancy: A Review of the Literature. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme. 2019; 51: 770-778.
- Lum G (2011) Falsely Elevated Parathyroid Hormone-Related Protein (PTHRP) in a Patient With Hypercalcemia and Renal Failure. Lab Med. 2011; 42: 727–728.
- Williams HJ, Davies AM, Chapman S. Bone within a bone. Clinical radiology. 2004; 59: 132-144.