
Case Report
J Pediatri Endocrinol. 2022; 7(2): 1055.
Effect of the Use of GnRH Analogs in Low-Grade Cerebral Glioma
De Lucio Delgado A¹*, Villegas Rubio JA¹, Riaño Galán I² and Pérez Gordón J²
¹Department of Oncology Pediatric, Hospital Universitario Central de Asturias, Spain
²Department of Endocrinology Pediatric, Hospital Universitario Central de Asturias, Spain
*Corresponding author: De Lucio Delgado A, Department of Oncology Pediatric, Hospital Universitario Central de Asturias, 33011 Oviedo, Spain
Received: October 9, 2022; Accepted: November 10, 2022; Published: November 17, 2022
Abstract
Low-grade gliomas are children’s most common brain tumors, presenting a wide range of clinical, histological, and biological behavior. Estrogens and progesterone are steroid hormones. In recent years, there has been speculation about its relationship with its role in the development of tumors. We describe the case of a 2-year-old girl with a low-grade brain tumor treated with chemotherapy without changes in its measurements. Initiated treatment with Decapeptyl® due to precocious puberty presents a decrease in its solid component, more than 50% of the initial size three years after starting treatment. Various studies have described the influence of estrogen and progesterone on the development of gliomas, decreasing or increasing their expression in those tumors of greater aggressiveness, respectively. Despite the study of the tumor-hormonal expression relationship in other types of tumors, in brain tumors, its role in their treatment remains to be known.
Keywords: Low-grade glioma; Child; Steroid hormones
Introduction
Brain tumors are the second leading cause of childhood cancer in children between 0 and 14 (approximately 22%) [1]. Low-Grade Gliomas (LGG) represent the most frequent group (30-50%) [2] constituting a very heterogeneous class of tumors in terms of the clinic, histology, and biological behavior. Pilocytic astrocytoma is the histological subtype most frequently found in this age group [3]. The annual incidence of GBG is close to 10-12 cases per million children under fifteen years of age, with a male: female ratio of 1.1:1/1.2:1.
The objective of this manuscript was to describe our experience with a pediatric patient with a low-grade glial tumor and her response to the use of gonadotropin-releasing hormone (GnRH) analog drugs.
Case Report
A 2-year-old and an 11-month-old girl consulted in the Pediatric Emergency Department of a tertiary care hospital due to persistent vomiting, gait disturbance, and striking instability of one week’s evolution. Given these symptoms, Computerized Tomography (CT) scan was performed, objectifying a mass in the supratentorial region with an essential component of hydrocephalus. The study is completed with a cranial MRI (single group located in the suprasellar area with a solid and cystic part), a hormonal study (FSH, LH, estradiol, progesterone, prolactin, ACTH, cortisol, IGF-I, IGFBP3, TSH, FT4, and FT3), and regular tumor markers (AFP and B-HCG), as well as standard visual and auditory potentials. A ventriculoperitoneal shunt was placed with improvement in the symptoms of intracranial hypertension, performing a biopsy of the lesion in the same surgical act. Pathological anatomy confirms the etiology of pilomyxoid astrocytoma (LGG, grade I) with KIAA1549- BRAF mutation. Chemotherapy started with weekly Vinblastine 6 mg/m2/dose for 70 weeks. This treatment ended in May 2019. In the image controls performed every three months, no changes were observed in terms of the volume of the lesion or its contrast uptake (except for a slight initial increase in the mass in the first months, explained by the antiangiogenic activity of vinblastine that can produce an initial growth during the first months of treatment followed by a subsequent reduction in tumor size [4]). The tumor mass at the end of therapy was described with similar measurements before the start of treatment.
Hormonal analytical controls requested every 3-4 months show normal values. However, two years and one month after starting Vinblastine, an increase in LH is observed (from 1.9 U/L (normal range <0.1-0.5 U/L) rises to 6.7 U/L five months later FSH values also increase from 5.6 U/L to 7.5 U/L (normal range 0.2-11.1), estradiol from 8.6 pg/ml to 17.3 pg /mL (normal range for patient age < 5 pg/mL) and progesterone from 0.05 to 0.46 ng/mL (normal range for patient age < 0.05 ng/mL) (Figure 1). The rest of the hormonal study (thyroid hormones, prolactin, DHEA, androstenedione, total testosterone, free testosterone, ACTH, cortisol), as well as the blood count and biochemistry, show values within the established parameters. The anthropometric data, physical examination, and other complementary tests of the patient in May of that year are Weight: 20.8 Kg (+1.11 SD), Height: 114.5 cm (+2.06 SD); TA: 99 /53 (p59/p40); Growth rate: 19.6 cm/year (+15.13 SD) with 2.2 cm of growth in the last month and a half. Presence of bilateral breast button, no axillary, pubarche stage I. Normal female external genitalia. Bone age: close to 6 years of bone age for a chronological age of 4 and four months. Pelvic ultrasound for evaluation of sexual appendages: according to the patient’s age. The studies are completed with the LH-RH test, which shows values compatible with activation of the pituitary-gonadal axis (FSH at 0 minutes of 4.8 U/L, 30 minutes of 18 U/L, and 60 minutes of 17.9 U/L; LH at 0 minutes 3.8 U/L, 30 minutes: 50.5 U/L and at 60 minutes 36.8 U/L). Given the reported data of precocious puberty, treatment with triptorelin, a gonadotropin-releasing hormone analog (Decapeptyl®), was started in July 2019 at a dose of 300 mcg/dose i.m. every 12 weeks. Despite the virtual normalization of FSH and LH values, it continues with an important growth rate, requiring dose adjustment to 128 mcg/Kg/ dose every 21 days.
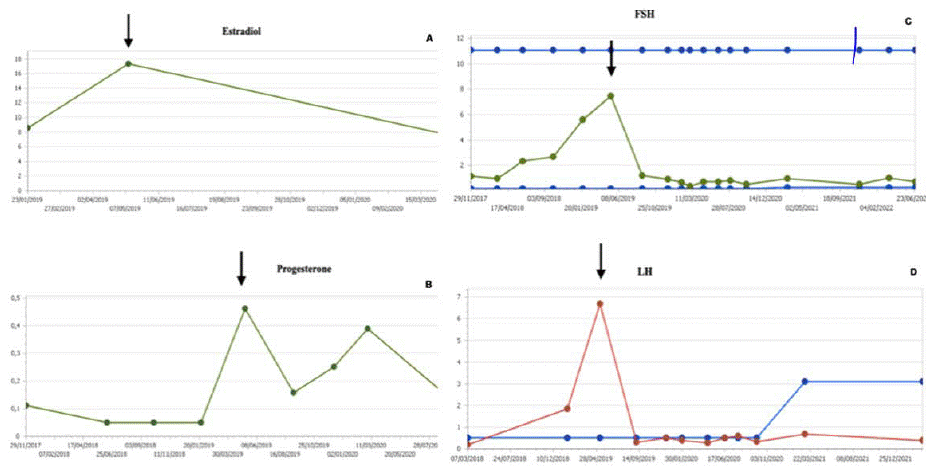
Figure 1: Evolution of estradiol (A), progesterone (B), FSH (C), and LH (D) values before and after starting hormonal treatment (July 2019).
Regarding hormonal controls, IGF-1 and IGFBP3 values show a progressive increase, with an altered response to repeated oral glucose overload tests. Given the suspicion of failure in the growth hormone axis, treatment with octreotide acetate (Sandostatin Lar 10®) 1/2 vial i.m. was started in September 2021every 28 days, being able to space the Decapeptyl every ten weeks. Latest anthropometric data and physical examination at 7 years and 5 months: Weight: 32.5 Kg (+1.15 SD), Height: 138.6 cm (+2.66 SD), Growth rate: 4.7 cm/year (p18, -0.95 SD), TA 97/65 (p30/p63). Thelarche 1, no axillary. Pubic hair on the labia majora, not on the mons pubis.
In the Magnetic Resonance Imaging (MRI) control two months after starting treatment with Decapeptyl®, a decrease in the solid component of the tumor was observed with a significant reduction in contrast uptake. In successive controls, this decrease results in more than 50% of the initial size without contrast enhancement (last image June 2022) (Figure 2). The Pathological Anatomy Service was requested to review the preserved tumor tissue from the diagnosis to verify the presence or absence of estrogen and progesterone receptors using immunohistochemical techniques, resulting in negative results.
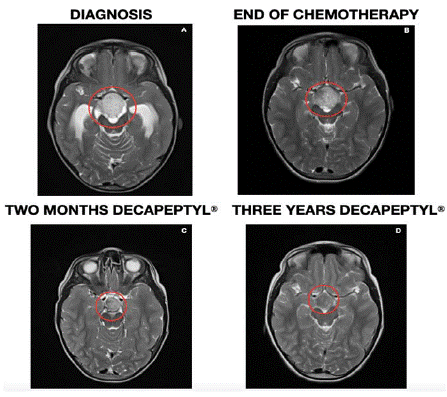
Figure 2: Magnetic resonance image at four different moments: at debut (A),
end of treatment (B), after two months of hormonal treatment (C), and current
image (D).
Discussion
Steroid hormones have a well-known neuroprotective function in neurological disorders like Parkinson’s disease or Alzheimer’s [5]. However, it was not until 1983 that the possible relationship of these hormones with the development of brain tumors was described for the first time.
Several studies have described the influence of estrogens on the development of gliomas through direct interaction with their receptors (ER-a and ER-Β) or the activation of potentially oncogenic mediators. Both ER isoforms are expressed in astrocytomas, with ER-a predominating. The presence of both ERs decreases in those astrocytomas with higher degrees of malignancy, thus suggesting their neuroprotective role [6]. These findings and a higher incidence of gliomas in men and postmenopausal women indicate that estrogens could reduce tumor proliferation7. However, several studies published by González-Arenas et al. have shown that estradiol “in vitro” induces the growth of astrocytes through its interaction with ER-a and its regulation of gene expression involved in the cell cycle, angiogenesis, and metástasis [7]. Selective estrogen receptor modulators could explain all these results (SERMs) that can function as estrogen antagonists or agonists, depending on the target tissue and the ER subtype, thus mediating a specific response. This way, individual SERMs can act as pure agonists, antagonists, or mixed agonists/antagonists [8]. In our patient, the association of the start of Decapeptyl with the decrease of the solid component of the tumor seems clear, even with the expression of negative markers in the sample studied. This could be since the presentation of the ER is heterogeneous in the tumor tissue, not having been detected due to the scarcity of the piece (diagnostic biopsy, not tumor resection).
Progesterone also has a known role in neuronal excitability, learning, and the proliferation of brain tumors. The best-known association is with meningiomas [9]. It seems that, unlike estrogens, the expression of PR increases with the histological malignancy of astrocytomas. In vitro studies indicate that progesterone promotes cell proliferation in these tumors and the face of genes that are important for tumor growth and spread. As with estrogens, other studies suggest that this hormone has antiproliferative properties and apoptotic effects in ovarian, breast, endometrial, and colon tumors, as well as in gliomas [10].
Conclusions
Treatment with Decapeptyl® allowed this patient to achieve an apparent reduction in the size of her tumor not obtained with chemotherapy treatment, without side effects, and with good tolerance to it. This response is promising today, not knowing for the moment what will happen when it is completed. More extensive studies in the pediatric population would be necessary to obtain more reliable data on the effectiveness of hormonal treatment in this type of tumor and assess the situation once treatment is discontinued and the appearance of long-term side effects.
References
- Cifras básicas del cáncer infantil. Cáncer infantil en España: Preguntas y datos. Registro Nacional de tumores infantiles RETI- SEHOP; [Internet]. España: RETI-SEHOP; actualizado octubre 2014 [citado el 19 de septiembre de 2022]. Disponible en: https://www.uv.es/rnti/pdfs/B1.05.
- International Consortium on Low-Grade Glioma – ICLGG of the International Society of Pediatric Oncology – SIOP. Cooperative Multicenter Study for Children and Adolescents with Low-Grade GliomaSIOP - LGG. 2004.
- Broniscer A, Baker SJ, West AN, Fraser MM, Proko E, Kocak M, et al. Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. Journal of Clinical Oncology. 2007; 25: 682–9.
- Massimino M, Spreafico F, Cefalo G, Riccardi R, Tesoro-Tess JD, Gandola L, et al. High response rate to cisplatin/etoposide regimen in childhood lowgrade glioma. J Clin Oncol. 2002; 20: 4209–16.
- Kabat GC, Etgen AM and Rohan TE. Do steroid hormones play a role in the etiology of glioma?. Cancer Epidemiol Biomark Prev. 2010; 10: 2421-7.
- Dueñas Jiménez JM, Candanedo Arellano A, Santerre A, Orozco Suárez S, Sandoval Sánchez H, et al. Aromatase and estrogen receptor alpha mRNA expression as prognostic biomarkers in patients with astrocytomas. J Neurooncol. 2014; 119: 275-84.
- Carroll RS, Zhang J, Dashner K, Sar M, Black PM. Steroid hormone receptors in astrocytic neoplasms. Neurosurgery. 1995; 37: 496-503; discussion 503-4.
- González-Arenas A, Peña-Ortiz MA, Hansberg-Pastor V, MarquinaSánchez B, Baranda-ávila N, et al. PKCa and PKCd Activation Regulates Transcriptional Activity and Degradation of Progesterone Receptor in Human Astrocytoma Cells. Endocrinology. 2015; 156: 1010-22.
- Rosal AM, Da Silva BB. Evaluation of estrogen and progesterone receptors in the non-neoplasia breast tissue of women of reproductive age exposed to tamoxifen and raloxifene: a randomized, double-blind study. Breast Cancer Res Treat. 2011; 125: 797-801.
- Germán-Castelán L, Manjarrez-Marmolejo J, González-Arenas A, González- Morán MG, Camacho-Arroyo I. Progesterone induces the growth and infiltration of human astrocytoma cells implanted in the cerebral cortex of the rat. BioMed Res Int. 2014; 2014: 393174.