
Research Article
J Pediatri Endocrinol. 2024; 9(1): 1063.
Correlation Among Thyroid Hormone and Microvascular Complications in Euthyroid Patients with T2DM
Lei-Lei Cheng; Bo-Wei Liu; Fu-Zai Yin*
Department of Endocrinology, First Hospital of Qinhuangdao, Province People’s Republic of China
*Corresponding author: Fu-zai YiDepartment of Endocrinology, First Hospital of Qinhuangdao No. 258, Wenhua Road, Qinhuangdao, 066000 Hebei Province People’s Republic of China. Email: yinfuzai62@163.com
Received: January 17, 2024 Accepted: February 22, 2024 Published: February 29, 2024
Abstract
Objective: To investigate the relation among thyroid hormone and Diabetic Microvascular Complications (DMVC) in euthyroid patients with Type 2 Diabetes Mellitus (T2DM).
Methods: We retrospectively analysis was performed for 785 euthyroid patients with T2DM from June 2019 to August 2020 which were hospitalized in the Department of Endocrinology of the First Hospital of Qinhuangdao, and 289 patients had at least one DMVC. The prevalence of Diabetic Retinopathy (DR) was 15.16%, Diabetic Nephropathy (DN) was 31.08% and Diabetic Peripheral Neuropathy (DPN) was 29.30%. The effect of thyroid hormone on DMVC were compared.
Results: Logistic regression analysis showed that, low FT3 was an independent risk factor for DR and DN (OR=0.542, 95% CI: 0.357-0.825, P=0.004; OR=0.715, 95% CI: 0.523-0.977, P=0.035). No significant relation showed between FT4 TSH and DR DN.
Conclusion: Euthyroid patients with T2DM with retinopathy and/or nephropathy had lower FT3 concentrations, while no association between FT4 and TSH and diabetic microvascular complications was found. There may be a certain association between poor glycemic control, low FT3 concentration, and microvascular complications in patients with T2DM, and future studies need to confirm the causal relationship and exact mechanism.
Keywords: Thyroid hormone; Type 2 diabetes mellitus; Diabetes microvascular complications
Abbreviations: TH: Thyroid Hormone; DMVC: Diabetic Microvascular Complications; T2DM: Type 2 Diabetes Mellitus; DR: Diabetic Retinopathy; DN: Diabetic Nephropathy; DPN: Diabetic Peripheral Neuropathy; TSH: Thyroid-Stimulating Hormone; FT3: Free Serum Triiodothyronine; BMI: Body Mass Index
Introduction
Worldwide, about 540 million persons have diabetes [1]. Various complications occur during disease progression, such as Diabetic Retinopathy (DR), Diabetic Nephropathy (DN), Diabetic Peripheral Neuropathy (DPN), and macrovascular complications. As the disease progresses, various forms occur that lead to blindness, kidney failure, and even life-threatening conditions. Many studies on the pathogenesis of DMVC were conducted, but the exact mechanism and effective treatment need to be further explored.
Thyroid hormone receptors are present in vascular endothelial tissues throughout the body, so thyroid hormone concentration affects vascular lesions. Numerous clinical studies have confirmed effect abnormal thyroid function is associated with DMVC. However, the exact mechanism on the relationship between patients with normal thyroid function and DMVC studies and new strategies for treatment require further study.
Methods
Study Participants
785 T2DM patients with normal thyroid function were collected who were hospitalized in the Department of Endocrinology of the First Hospital of Qinhuangdao from June 2019 to August 2020, (436 men, 349 women, median age 57.02±13.82). Patients were excluded if they had type 1 or other types of diabetes, acute complications of uncorrected diabetes mellitus, a history of hypothalamic or pituitary disorder, thyroid malignant disease, dythyroidism, oral thyroid-related drug therapy, hepatic insufficiency (2.5 times the normal value of AST and ALT), malignancy, pregnancy or lactation, anemia and hypoproteinic dystrophy, acute infectious states, and ocular history affecting fundus photography, such as trauma, eye injections, and surgery. The Ethics Committee of Qinhuangdao First Hospital approved this study, and all enrolled patients signed informed consent.
Research Methods
Type 2 diabetes mellitus diagnostic criteria: Accord with the criteria of the American Diabetes Association [2]. Diabetic Retinopathy (DR) diagnostic criteria: All subjects underwent ophthalmograms and fundus photography [3]. Experienced ophthalmologists used the Obaur ultra-wide-angle laser scanning camera [4] to acquire digital retinal photographs, and diagnoses met the international diabetic retinopathy criteria [3]. Diabetic Nephropathy (DN) diagnostic criteria: After exclusion of renal organopathy and urinary tract infection, results were consistent with eGFR =60 ml/min/1.73 m² and/or ACR=30 mg/g [5]. Diabetic Peripheral Neuropathy (DPN) diagnostic criteria: Patients had at least two positive results in sensory symptoms, signs, or abnormal reflexes. Abnormal nerve conduction tests were defined as the presence of at least one abnormality (amplitude, incubation period, F-wave, or nerve conduction velocity) in two or more nerves between the median, peroneal, and sural nerves [6]. Diagnostic criteria for normal thyroid function: FT3(1.58-3.91pg/ml), FT4(0.70-1.48ng/dl) and TSH (0.35-4.94uIU/ml).
Participants were divided into DR (n=119), DN (n=244), and DPN (n=230) groups according to the presence or absence of diabetic microvascular complications. Clinical data such as sex, age, history of hypertension, and course of diabetes were collected. Height and weight were measured, Body Mass Index (BMI) was calculated, and systolic and diastolic blood pressure were measured.
After fasting for 8-12 hours, 5 ml of venous blood was drawn, and concentrations of the following entities were measured: glycosylated hemoglobin (HbA1c), fasting blood glucose, homocysteine, uric acid, cystatin C, cholesterol, triglycerides, low, high-density lipoprotein cholesterol, serum creatinine and calculated eGFR (CKD-EPI method). Urine microalbumin/urine creatinine; Fasting C-peptide; Antithyroid peroxidase antibody, thyroglobulin antibody, serum Free Triiodothyronine (FT3), serum Free Thyroxine (FT4) and Thyroid-Stimulating Hormone (TSH) were also measured (Chemiluminescence).
FT3 and FT4 were divided into AFT3, BFT3 and AFT4, BFT4 groups with the median as the cut-off point respectively. (The truncation values were 2.69 pg/ml and 0.98 ng/dl, respectively). TSH were used to divide the patients into ATSH and BTSH groups with 2.5uIU/ml as the cut-off point. The cut-off value for substandard HbA1c is set at 6.5% [2].
Statistical Processing
SPSS 23.0 software was used for statistical analysis. Normal distribution measurements are expressed as mean ± standard deviations, and independent sample t-tests are used for intergroup comparisons. Nonnormal distribution measurements are expressed as medians and two-percentile spacing [M (QL, QU)], Nonparametric tests are used. Counting data are expressed as n (%), using a chi-square test. Logistic regression was used to analyze the relationship between thyroid hormones and microvascular complications of diabetes.
Results
1.The comparison of general clinical data of each group of patients
2.The prevalence of microvascular complications at different thyroid hormone concentrations (Figure 1-3).
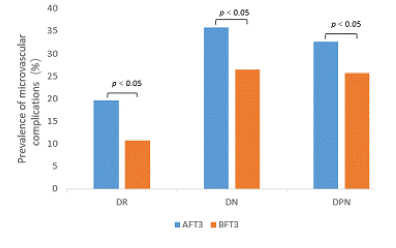
Figure 1:
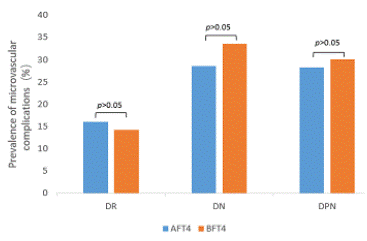
Figure 2:
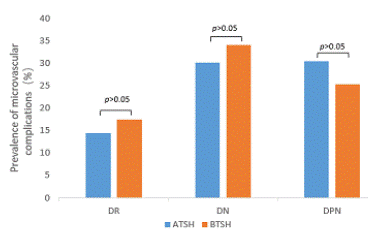
Figure 3:
The prevalence of DR was 19.6% and 10.8% (p<0.01), DN was 35.8% and 26.4% (p<0.01), and DPN was 32.7% and 25.7% (p<0.05), respectively, in the AFT3 and BFT3 groups. There was no significant difference between TSH and FT4 and the prevalence of each complication.
3. The incidence of low FT3 was 89.6% and 10.4%, respectively, in HbA1c=6.5% and HbA1c 6.5% groups (p<0.05) (Table 2).
DR
DN
DPN
Yes
No
Yes
No
Yes
No
age (y)
57.42±12.98*
54.31±14.04
57.94±14.55**
53.36±13.40
58.75±12.86**
53.15±14.02
Sex [male (%)]
64(53.8)
357(53.7)
131(53.7)
289(53.5)
126(54.8)
302(54.5)
BMI (kg/m2)
25.43±3.26
25.97±3.90
25.98±3.91
25.84±3.77
25.31±3.39**
26.12±3.95
Duration of T2DM (y)
14.22±8.09**
6.65±6.83
10.00±8.19**
6.80±7.01
10.42±8.47**
6.72±6.84
Duration of HBP (y)
5.71±8.60
4.51±7.25**
6.64±8.59**
3.82±6.75
5.70±8.27*
4.28±7.09
SBP (mmHg)
142.18±24.06
139.20±54.45
143.15±23.17
138.08±59.41
140.86±21.62
139.16±59.02
DBP (mmHg)
84.13±11.37
85.08±11.83
85.73±12.69
84.57±11.30
84.24±12.14
85.22±11.60
FPG (mmol/L)
8.14±3.48
8.83±4.03
9.17±4.18
8.53±3.83
8.89±4.13
8.67±3.88
HbA1c (%)
8.89±1.92
8.61±2.07
8.97±2.05**
8.51±2.04
8.92±2.02*
8.54±2.06
C-peptide (ng/ml)
1.26±0.94**
1.85±1.30
1.80±1.39
1.74±1.21
1.58±1.21**
1.84±1.29
Cys-C (mg/L)
1.32±0.79**
1.01±0.28
1.25±0.65**
0.97±0.21
1.19±0.63**
1.00±0.27
Hcy (umol/L)
14.18±6.82
13.63±6.52
15.08±8.21**
13.29±5.75
14.29±6.56
13.48±6.56
FT3 (pg/ml)
2.54±0.41**
2.71±0.41
2.58±0.44**
2.72±0.39
2.62±0.41*
2.71±0.41
FT4 (ng/dl)
0.98±0.10
0.99±0.12
1.01±0.12**
0.98±0.11
0.99±0.11
0.99±0.12
TSH (mIU/L)
3.65±0.48
3.47±0.50
1.96±1.01
1.90±0.96
1.84±0.96
1.94±0.98
FT3/FT4
2.61±0.48**
2.77±0.52
2.60±0.51**
2.81±0.50
2.67±0.50*
2.77±0.52
SPAINGD
28.86±5.33**
30.61±5.71
28.79±5.66**
31.04±5.57
29.59±5.53*
30.65±5.73
SPAINGT
2.67±0.92
2.85±1.29
2.85±1.30
2.80±1.21
2.88±1.19
2.79±1.25
UA (umol/L)
366.15±222.76
336.82±96.07
355.91±111.21*
334.61±129.7
347.56±174.84
338.80±96.87
TC (mmol/L)
5.31±1.57
5.27±1.29
5.37±1.54
5.23±1.23
5.34±1.54
5.25±1.24
TG (mmol/L)
2.33±1.57
2.47±2.57
2.81±2.86*
2.30±2.32
2.27±2.17
2.53±2.64
LDL-C(mmol/L)
2.79±0.94
2.93±0.87
2.91±0.94
2.91±0.85
2.93±0.99
2.90±0.84
HDL-C(mmol/L)
13±0.30
1.09±0.24
1.10±0.29
1.09±0.23
1.10±0.27
1.09±0.24
LPa (umol/L)
327.01±311.82*
240.18±244.51
270.43±278.80
243.80±242.45
291.50±297.51*
235.36±232.04
eGFR (ml/min/1.73m2)
98.32±34.84**
108.37±22.41
96.89±32.56**
110.84±18.20
98.17±26.87
109.84±22.62
ACR (mg/mmol)
16.27±21.36**
4.19±8.79
16.40±17.84**
1.16±0.59
10.02±16.64
4.35±9.54
Table 1: Compared with the group without microvascular complications, FT3 level were negatively correlated with the prevalence of DR, DN, and DPN, and FT4 level were negatively correlated with DN prevalence; the differences were statistically significant (p<0.05). TSH level was not associated with any microvascular complications. The prevalence of DN was positively correlated with homocysteine concentration (p<0.05).
HbA1c = 6.5%
HbA1c < 6.5%
c2
p
The incidence of low FT3 (%)
89.6
10.4
9.09
0.003
Table 2:
4. Logistic regression analysis of the influencing factors of microvascular complications in patients with T2DM
In Model 1, we found that FT3 was negatively correlated with all three microvascular complications, and in Model 2, we adjusted for traditional risk factors such as age, T2DM disease course, BMI, HbA1c and FPG and found that FT3 was negatively correlated with DR and DN, that is, lower levels of FT3 in the normal range were independent risk factors for DR and DN (Table 3-5).
FT3 n (%)
Model 1
Model 2
OR (95%CI) p
OR (95%CI) p
AFT3 76(63.87)
BFT3 43(39.13)0.499(0.333-0.747)0.001
10.542(0.357-0.825)0.004
1
Table 3: FT3 and DR.
FT3 n (%)
Model 1
Model 2
OR (95%CI) p
OR (95%CI) p
AFT3 139(56.97)
BFT3 105(43.03)0.644(0.475-0.874) 0.005
10.715(0.523-0.977) 0.035
1
Table 4: FT3 and DN.
FT3 n (%)
Model 1
Model 2
OR (95%CI) p
OR (95%CI) p
AFT3 127(55.46)
BFT3 102(44.54)0.711(0.522-0.968) 0.030
10.807(0.586-1.111) 0.807
1Note: Model 1 means Univariate logistic regression analysis
Model 2 means Multivariate logistic regression analysis
Table 5: FT3 and DPN.
Discussion
Diabetic microvascular complications are common, and they negatively affect the quality of life of patients with diabetes. The mechanism by which hyperglycemia induces microvascular complications is complex, and the course of diabetes, dyslipidemia, age, BMI, homocysteine, and thyroid dysfunction are all risk factors. Controlling its progression is an important goal of improving patient clinical outcomes. In this study, we found that in euthyroid patients with T2DM, low FT3 was an independent risk factor for DR and DN, but not related to DPN. FT4 and TSH were not associated with any of the three complications, which may be related to the metabolic process of these two hormones in the body, that is, FT3 is the hormone that ultimately acts on various organs, rather than FT4 and TSH.
In animal experiments, Énzsöly et al. found [7] that, when the expression of external and medium wavelength-sensitive cones (M-cones) and short-wavelength-sensitive cones (S-cones) was out of balance, color discrimination ability to distinguish color was directly impaired [8]. These findings were confirmed in subsequent human trials, and studies have also confirmed that the imbalance in the expression of bicones is directly related to thyroid homeostasis. The conversion of thyroxine to triiodothyronine (T4 to T3) decreases with the weakening of peripheral deiodidase activity [9]. Triiodothyronine directly increases pancreatic ß cell activity and controls insulin secretion and intracellular glucose availability, through which FT3 can affect vascular endothelial function. Our study showed that even subtle changes in serum thyroid hormone concentration within the physiological range can have these consequences, which is consistent with the findings of Zou [10] et al. that FT3 concentration of euthyroid patients with type 2 diabetes mellitus are inversely associated with DR. In addition, photoreceptors release inflammatory factors, such as COX2, ICAM-1, and iNOS, which leads to endothelial cell damage that, in turn, triggers fundus vascular lesions [11,12].
Thyroid hormones are involved in many physiological functions during the growth and development of the kidneys. The kidneys not only metabolize and eliminate thyroid hormones, but the kidneys are also regulated by some thyroid hormones. Thyroid dysfunction can cause changes in renal blood flow, eGFR, tubular absorption and secretion, and even changes in renal structure [13]. Numerous studies have confirmed that low FT3 is strongly associated with endothelial dysfunction in patients with kidney disease [14,15]. Decreased response of endothelial cells to nitric oxide was found in patients who had both diabetic nephropathy [DN] and hypothyroidism. This decreased response was possibly due to the accumulation of nitric oxide inhibitors [16] and decreased nitric oxide availability [17], impairment of endothelial function, with direct impairment of reduced glomerular filtration rate and increased urinary albumin. The endogenous nitric oxide system inhibitor asymmetric di-Methylarginine (ADMA) is a major participant in endothelial dysfunction in patients with DN and is also involved in the pathophysiological processes of oxidative stress [18]. Interestingly, administration of thyroxine to mice, improved the phenomenon significantly [19]. On the other hand, the expression of 3,5-deiodidase in the kidneys affects the metabolism of thyroid hormones in the kidneys, which suggests that sensitivity to thyroid hormones is also reduced due to decreased kidney function [20]. Our findings suggest that, in people with normal thyroid function, FT3 concentration is inversely proportional to the prevalence of DN and not associated with TSH. These findings do not agree with findings of Han et al. [21], although these conflicting results may be due to different characteristics of participants.
Homocysteine promotes systemic inflammation, oxidative stress, decreased nitric oxide availability, insulin resistance, and endothelial dysfunction, and then leads to angiopathy in T2DM. Abnormal concentrations of homocysteine, folic acid, and vitamin B12 are risk factors for diabetic nephropathy [22]. In a study of the relation between thyroid function and homocysteine in pregnant women [23], Hammouda et al. found that TSH was positively correlated with serum homocysteine concentration. In our study, FT3 in serum in euthyroid patients with diabetic nephropathy was negatively correlated with homocysteine concentration. Therefore, we speculate that euthyroid patients with DN can be administered folic acid or vitamin B12 to alleviate the progression of DN.
DPN is a large clinical disease; its prevalence among T2DM patients is about 45% [24]. The main pathological characteristics of DPN are axial mutation and segmental demyelination caused by hyperglycemia. Clinicians often recognize DPN later than retinopathy and nephropathy, which leads to serious consequences. Diabetic peripheral neuropathy patients with early paresthesias are usually progressive, including sock signs and glove signs of the extremities, and later may appear as proximal limb numbness, weakness and atrophy [25], There may even be erectile dysfunction and lower urinary tract symptoms in men; thus, it is especially important to strengthen early blood sugar control. The pathogenesis of DPN is still uncertain, and the metabolic and microvascular factors that cause mutation of the nerve fiber axis have been the more clinically studied [6]. Nerve conduction velocity and amplitude are negatively affected in patients with thyroid dysfunction [26]. Impaired vascular endothelial function in T2DM patients may be a factor in progression of DPN, whereas nitric oxide regulates vasodilation, thereby alleviating endothelial dysfunction [27]. Vicinaza et al. suggested that the endothelial cell nitric oxide-producing process can be mediated by thyroid hormones [28]. Thyroid hormone is essential for development of the Central Nervous System (CNS) in the fetal and neonatal stages and for the maintenance of the structure and function of the CNS in adults. Notably, the active form of FT3 specifically bound to various thyroid hormone receptors controls the complex hierarchical cascade of target genes that regulate activities such as the expression of Kruppel-like factor 9, one of the genes associated with initiating myelination [29].
Oxidative stress, inflammation, and mitochondrial dysfunction lead to changes in the pathological processes of DPN, and TSH regulates these processes by stimulating the thyroid gland to release T3 in conjunction with thyroid hormone receptors. A proper increase in T3 can reduce TSH, mobilize more mitochondrial production, and produce more ATP, thereby combating processes such as inflammation and oxidative stress and slowing the progression of DPN [30]. Han et al. [31] reported that TSH concentrations in patients with T2DM were independently associated with DPN (OR = 1.87, P = 0.014) after adjusting for confounding factors, which disagrees with our findings, we think this may be related to the different amounts of iodine intake in coastal and inland areas.
There are some limitations in our article. The study was a single-center, cross-sectional study; multi-center, long-term prospective studies are needed to reveal the mechanism of action. We did not exclude patients in the autoimmune state, and further studies need to improve the data of thyroid-related antibodies and rT3.
Conclusion
Euthyroid patients with T2DM with retinopathy and/or nephropathy had lower FT3 concentrations, while no association between FT4 and TSH and diabetic microvascular complications was found. There may be a certain association between poor glycemic control, low FT3 concentration, and microvascular complications in patients with T2DM. This study may provide a basis for prospective cohort studies of the pathogenesis of diabetic microvascular complications and ideas for examining treatment options for diabetic vascular complications. Future studies need to confirm the causal relationship and exact mechanism.
Author Statements
Acknowledgments
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services.
References
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022; 183: 109–19.
- Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021; 44: 15–33.
- Fei G, Hanyi M. Diagnosis and treatment of diabetic retinopathy. Chinese Journal of Clinicians. 2021; 49: 1402–4.
- Li Z, Wang H. Obaur daytona laser scanning ophthalmoscope in the screening of patients with diabetic retinopathy. Medical equipment. 2018; 31: 127–8.
- Qi C, Mao X, Zhang Z. Classification and Differential Diagnosis of Diabetic Nephropathy. J Diabetes Res. 2017; 2017: 8637138.
- Martin CL, Albers JW, Pop-Busui R. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014; 37: 31–8.
- Enzsoly A, Hajdú RI, Turóczi Z, Szalai I, Tatrai E, et al. The predictive role of thyroid hormone levels for early diabetic retinal changes in experimental rat and human diabetes. Invest Ophthalmol Vis Sci. 2021; 62: 20.
- Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN jr. UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci. 1999; 19: 442–55.
- Mendoza A, Hollenberg AN. New insights into thyroid hormone action. Pharmacol Ther. 2017; 173: 135–45.
- Zou J, Li ZP, Tian F, Zhang Y, Xu C. Association between normal thyroid hormones and diabetic retinopathy in patients with type 2 diabetes. Biomed Res Int. 2020; 2020: 8161797.
- Kern TS. Do photoreceptor cells cause the development of retinal vascular disease? Vision Res. 2017; 139: 65–71.
- Tonade D, Liu H, Palczewski K, Kern TS. Photoreceptor cells produce inflammatory products that contribute to retinal vascular permeability in a mouse model of diabetes. Diabetologia. 2017; 60: 2111–20.
- Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol. 2009; 160: 503–15.
- Ellervik C, Mora S, Ridker PM, Chasman DI. Hypothyroidism and Kidney Function: A Mendelian Randomization Study. Thyroid. 2020; 30: 365–79.
- Papadopoulou A-M, Bakogiannis N, Skrapari I, Moris D. Chris Bakoyiannis .Thyroid Dysfunction and Atherosclerosis: A Systematic Review. Vivo. 2020; 34: 3127–36.
- Yilmaz M, Sonmez A, Karaman M, Ay SA, Saglam M. Low triiodothyronine alters flow-mediated vasodilatation in advanced nondiabetic kidney disease. Am J Nephrol. 2011; 33: 25–32.
- Stefanowicz-Rutkowska MM, Baranowska-Jurkun A, Matuszewski W, Em B-S. Thyroid dysfunction in patients with diabetic retinopathy. Endokrynol Pol. 2020; 71: 176–83.
- Tain YL, Hsu CN. Toxic dimethylarginines: asymmetric dimethylarginine (ADMA) and symmetric dimethylarginine (SDMA. Toxins (Basel. 2017; 9: 92.
- Napoli R, Guardasole V, Angelini V, Zarra E, D T. Acute effects of triiodothyronine on endothelial function in human subjects. J Clin Endocrinol Metab. 2007; 92: 250–4.
- Mantzouratou P, Lavecchia AM, Novelli R, Xinaris C. Thyroid hormone signalling alteration in diabetic nephropathy and cardiomyopathy: a “switch” to the foetal gene programme. Curr Diab Rep. 2020; 20: 58.
- Han Q, Zhang J, Wang Y, Li H, Zhang R. Thyroid hormones and diabetic nephropathy: An essential relationship to recognize. Nephrology (Carlton. 2019; 24: 160–9.
- Mursleen M, Riaz S. Implication of homocysteine in diabetes and impact of folate and vitamin B12 in diabetic population. Diabetes Metab Syndr. 2017; 11: 141–6.
- Hammouda S, Mumena WA. Reduced serum concentrations of vitamin B12 and folate and elevated thyroid-stimulating hormone and homocysteine levels in first-trimester pregnant Saudi women with high A1C concentrations. Nutr Res. 2019; 72: 105–10.
- Roman-Pintos LM, Villegas-Rivera G, Rodríguez-Carrizalez AD, Miranda-Díaz AG, Cardona-Muñoz EG. Diabetic polyneuropathy in type 2 diabetes mellitus: inflammation, oxidative stress, and mitochondrial function. J Diabetes Res. 2016; 2016: 3425617.
- Castelli G, Desai KM, Cantone RE. Peripheral neuropathy: evaluation and differential diagnosis. Am Fam Physician. 2020; 102: 732–9.
- Zhu FF, Yang LZ. The Association between the levels of thyroid hormones and peripheral nerve conduction in patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2018; 126: 493–504.
- Lu M, Chong-Bo Y, Ling G, Jia-Jun Z. Mechanism of subclinical hypothyroidism accelerating endothelial dysfunction (Review. Exp Ther Med. 2015; 9: 3–10.
- Vicinanza R, Coppotelli G, Malacrino C, Nardo T, Buchetti B. Oxidized low-density lipoproteins impair endothelial function by inhibiting non-genomic action of thyroid hormone-mediated nitric oxide production in human endothelial cells. Thyroid. 2013; 23: 231–8.
- Zhang M, Ziyi M, Haochen Q, Zhongxiang Y. Thyroid hormone potentially benefits multiple sclerosis via facilitating remyelination. Mol Neurobiol. 2016; 53: 4406–16.
- Mancini A, Segni CD, Raimondo S, Olivieri G, Silvestrini A. Thyroid hormones, oxidative stress, and inflammation. Mediators Inflamm. 2016; 2016: 6757154.
- Han C, Xue H, Xinghai X, Yongze L, Xiaoguang S. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PLoS One. 2015; 10: 0135233.