
Research Article
J Pediatr & Child Health Care. 2016; 1(1): 1005.
A Survey of Pneumococcal Prophylaxis Practices among Sickle Cell Providers
Clay ELJ1,2, Hsu G³, Jordan L4, Xu H5 and Pace BS1*
1Department of Pediatrics, Augusta University, Augusta, GA, USA
2Department of Emergency Medicine, Augusta University, Augusta, GA, USA
3Department of Neonatology, Hospital of Ancona, Italy
4University of Miami, Miami, FL, USA
5Department of Biostatistics and Epidemiology, Augusta University, Augusta, GA, USA Co-first authors: Clay ELJ and Hsu G
*Corresponding author: Pace BS, Department of Pediatrics, Augusta University, Augusta, GA, USA
Received: June 07, 2016; Accepted: June 19, 2016; Published: June 21, 2016
Abstract
Aim: To identify the most common antibiotics used for pneumococcal prophylaxis among pediatric hematologists caring for individuals with Sickle Cell Disease (SCD).
Methods: A questionnaire was distributed to pediatric hematologists attending the 8th Annual Foundation for Sickle Cell Disease Research and Educational Symposium in Miami, FL. Information was collected related to provider characteristics, practice site setting, and antibiotics prescribed for pneumococcal prophylaxis in children with SCD. The data was analyzed for timing of initiation and cessation of antibiotic prophylaxis, antibiotic regimen of choice, and practice setting among participants.
Results: Sixty-two practitioners of mixed clinical characteristics (geographic location, monthly patient volumes, and academic versus private practice setting) who care for children with SCD completed the survey. The majority of hematologists practiced in the Midwest or Eastern United States. Of the total participants, 90.3% (56 hematologists) prescribed antibiotic prophylaxis to their patients while 9.7% did not. Of the practitioners who prescribed antibiotics, 80.6% utilized penicillin as the primary treatment for pneumococcal prophylaxis, while 9.7% used amoxicillin and the remaining 11.3% of hematologists used a variety of other antibiotics. The vast majority of infants started antibiotic prophylaxis by 3 months of age and discontinued treatment by 5 years. Furthermore, the impact of practice setting suggest a tendency of private practice hematologists to used amoxicillin as the first-line drug for pneumococcal prophylaxis compared to physicians in academic settings.
Conclusion: This study demonstrates variations in drug treatment practices for pneumococcal prophylaxis among hematologists caring for children with SCD.
Keywords: Sickle Cell Disease; Penicillin; Amoxicillin; Streptococcus pneumoniae; Pneumococcal Prophylaxis
Abbreviations
SCD: Sickle Cell Disease; HbS: Hemoglobin S; Streptococcus pneumoniae: S.pneumoniae; US: United States; PROPS-I: Prophylaxis Penicillin Study-I; PCN: Penicillin; Amox: Amoxicillin; yrs: years
Introduction
Sickle Cell Disease (SCD) is a group of inherited blood disorders produced by mutations in the β-globin gene located on chromosome 11. Various missense mutations involving the 6th codon of the β-globin gene result in abnormal globin chain variants with Hemoglobin S (HbS) being the most common. Individuals affected with homozygous βS-globin mutations (HbSS) or heterozygous for the sickle mutation and β◦-thalassemia (HbSβ◦-thalassemia) have a more severe disease phenotype [1,2]. They are at higher risk for developing complications compared to individuals with milder forms of SCD such as HbSC [3,4] and HbSβ+-thalassemia [5].
The hallmark of SCD is HbS polymerization, red blood cell sickling, vaso-occlusion and tissue ischemia. The most common manifestation of vaso-occlusion is severe reoccurring painful episodes. Additionally, patients with SCD have nutritional deficiencies, splenic dysfunction, and increased susceptibility to infection placing them at risk for multi-organ damage and failure [2,6,7]. Since several organ systems are affected by SCD, a multidisciplinary approach to patient care is recommended to produce the greatest impact on morbidity and long-term survival. Recent guidelines for comprehensive care were published in 2014 by the National Heart Lung and Blood Institute “Evidence-Based Management of Sickle Cell Disease” [8]. Advances in treatment options and standardization of health supervision and surveillance practices, have improved the average lifespan with the majority of persons with SCD surviving well into adulthood [9]. To promote further progress in health care delivery, it is imperative that hematologists and general care providers understand the complex nature of SCD and deliver the current standards of care to this population thus decreasing complications and improving quality of life.
Young children with SCD are at high risk for significant morbidity and mortality [1] related to sepsis in the first 3 years of life if not treated aggressively [2]. Sickle cell patients have altered immune responses putting them at risk for invasive infections due to Streptococcus pneumoniae (S.pneumoniae) and other encapsulated bacteria. The common loss of spleen function in the first year of life, deficiencies in complement and immunoglobulin production as well as delays in the clearance of invasive pathogens contribute to increased risk of invasive infections [10-14]. The landmark Prophylaxis Penicillin Study-I (PROPS-I) and Cooperative Study of Sickle Cell Disease [15,16] established the role of early diagnosis to identify babies with SCD and the routine use of prophylactic penicillin in the standard of care for infants with SCD. It was demonstrated an 84% reduction in the incidence of infection and no deaths from pneumococcal septicemia in children treated with penicillin [15]. A follow up study PROPS-II [17] demonstrated no significant difference in the percent of positive cultures for S.pneumoniae in those patients given penicillin prophylaxis after 5 years of age compared with the placebo group. These data suggest no additional benefit of continuing penicillin treatment after 5 years of age.
In the most recent guidelines “Evidence-Based Management of Sickle Cell Disease” the following recommendations were made for standards of care practices
1) Oral penicillin prophylaxis for children with sickle cell anemia (HbSS and HbSβ◦-thalassemia) until 5 years of age, 2) Vaccination against S.pneumoniae and other encapsulated organisms, and 3) Proper education regarding increased surveillance and early intervention in the event of fever or signs of infection [8]. Based on findings in the PROPS-II study [17], recommendations were given to discontinue penicillin prophylaxis at 5 years of age for children with an intact spleen or no history of invasive S.pneumoniae disease. Recommendations to consider withholding penicillin prophylaxis in patients with HbSC and HbSβ+-thalassemia were also included. Despite the established guidelines and clinical evidence for effectiveness, our survey data suggest up to 9.7% of infants do not receive preventative treatment against infection. Our study illustrates variations in the first-line antibiotic prescribed for S.pneumoniae prophylaxis among hematologists caring for patients with SCD. The implications of these practices and the potential effects of long-term antibiotic prophylaxis will be discussed.
Material and Methods
Survey design - A survey was completed by attendees of the 8th Annual Foundation for Sickle Cell Disease Research and Educational Symposium in 2014. The survey was composed of 10 closed-end questions about the routine care rendered to children with SCD in their practice. The data were collect using Survey Monkey Copyright© 1999-2015. The questions were designed to collect information regarding the provider’s practice location, practice setting, patient volume, and routine practice for S.pneumoniae prophylaxis (Pneumococcal Prophylaxis Practices among Sickle Cell Providers; www.surveymonkey.com). A total of 62 pediatric hematologists caring for patients with SCD of mixed clinical backgrounds responded to the survey.
Statistical analysis
After collection of the raw data, the Fisher’s exact test was conducted to determine correlations between prophylaxis regimen preferences and provider practice settings. The data were summarized as the Mean ± Standard Deviation and p-value <0.05 was considered a statistically significant difference.
Results
Characteristics of survey participants
The purpose of the survey was to identify variations in antibiotic prescribing practices for S.pneumoniae prophylaxis among hematologists caring for children with SCD. The survey was completed by attendees of the 8th Annual Foundation for Sickle Cell Disease Symposium, which is one of the largest gatherings of physicians caring for people with SCD. Out of 501 registered attendees, 62 pediatric hematologists completed the survey. The opportunity for attendees to complete the questionnaire was announced and a link was provided during the meeting. In addition for a limited time after the meeting, registered attendees could complete the survey online. The answers were recorded via Survey Monkey with an average completion time of 5 minutes per participant. The raw data from the surveys were compiled for statistical analysis.
The demographics of pediatric hematologists participating in the survey from several geographic locations in the United States (US) and internationally are shown in (Figure 1). The majority of the participants were from the Midwest and Eastern US regions. The characteristics of the clinical practice settings are summarized in Table 1. The majority of hematologists practiced within the continental US (91.9%) and 8.1% were from international practices. Analysis of the entire cohort revealed a wide variation of monthly patient volumes ranging from <50 patients for 43.5% to providers caring for >150 patients with SCD. These data suggest we captured a reasonable representation of hematologists with significant SCD patient loads in different practice settings.
Practice Characteristics of Providers
Practice setting United States
Practice setting International
91.9%
8.1%
Sickle cell patient volume: 0-50
Sickle cell patient volume: >50-100
Sickle cell patient volume: >100-150
Sickle cell patient volume: >150
43.5%
27.4%
11.3%
17.7%
Table 1: Practice characteristics of providers.
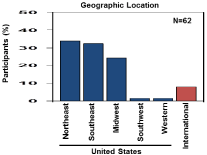
Figure 1: Geographic location of hematologists participating in the survey.
Shown is the distribution of hematologists by US regions and international
locations completing the online survey.
Some Hematologists Fail to Offer S.pneumonia Prophylaxis
To assess overall compliance among pediatric hematologists with the current recommendations for penicillin prophylaxis to prevent invasive S.pneumoniae infection [8,15,16] a series of queries were administered. The first question asked participants, do you prescribe S.pneumoniae prophylaxis? The vast majority of hematologists (90.3%) prescribed pneumococcal prophylaxis as recommended, however 9.7% failed to offer this treatment option to at-risk patients with SCD (Figure 2A). Of those offering prophylaxis, we next asked which antibiotic was prescribed to assess adherence with the recommendation of penicillin as the drug of choice [8,15]. In accordance with current standards of care, 80.6% of providers prescribed penicillin as the first-line drug with amoxicillin (9.7%) as the second most commonly prescribed agent (Figure 2B). Of note, 11.3% reported using “other” antibiotics however no participate identified the alternative drug used. However, for children with penicillin allergies, erythromycin would be a recommended alternative for S.pneumoniae prophylaxis. We next asked about common practices for the age of initiating and discontinuing prophylaxis treatment. Although there were considerable variations, 77.4% of US providers started antibiotic treatment between 0-3 months of age for infants with sickle cell anemia (Figure 3A). Of concern was the fact that 8.1% of providers initiated treatment after 9 months of age during which time infants are at high risk for invasive S.pneumonia disease. The next survey question asked about the age of discontinuing antibiotic treatment. Figure 3B demonstrated that once prophylaxis was initiated, 71.0% of providers discontinue penicillin or amoxicillin at 5 years of age according to current recommendations [8,16]. By contrast, 27.4% of children remained on antibiotic treatment after 5 years of age.
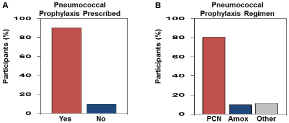
Figure 2: S. Pneumoniae prophylaxis prescribing practices of pediatric
hematologists.
A) Shown is the percentage of practitioners that prescribe antibiotic
prophylaxis to infants diagnosed with SCD by newborn screening or other
methods. B) The distribution of the different antibiotics prescribed for S.
pneumoniae infection prevention. Abbreviations: PCN: Penicillin, Amox:
Amoxicillin.
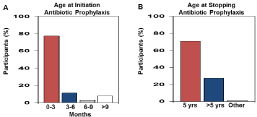
Figure 3: Age of prophylactic antibiotic initiation in children with SCD. A) The
majority of infants with SCD are treated with antibiotics by 3 months of age. A
significant number of infants go unprotected against the risk of overwhelming
sepsis up to 9 months. B) Shown is the distribution of ages in years (yrs)
when prophylaxis antibiotics are discontinued in children with SCD.
Practice setting is associated with choice of S.pneumoniae prophylaxis
In our final analysis for US hematologist only, we evaluated the impact of provider practice setting on clinical prescribing habits. The sample size of international providers limited our ability to conduct a similar analysis for that group. As shown in (Figure 4A), US providers (n=56) prescribed penicillin for 90% of infants with SCD as recommended. By contrast, amoxicillin was prescribed for 8.9% of patients suggesting US provider used other antibiotics less often when compared to entire group of hematologists where 11.3% used amoxicillin (Figure 2B). The last question addressed the impact of practice site setting on the choice of antibiotic prescribed. We observed 92% of academic compared to private hematologists used penicillin (p=0.049) as the first-line antibiotic (Figure 4B). By contrast, 40% of private practice hematologists used amoxicillin supporting a significant association between the primary choice of S.pneumoniae prophylaxis and practice setting among US hematologists.
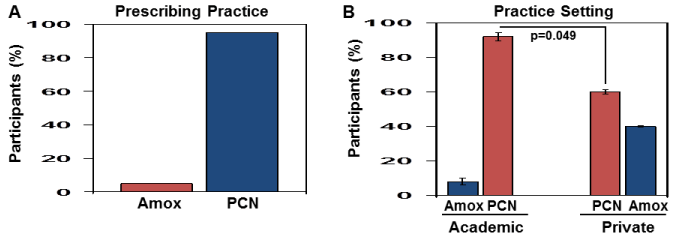
Figure 4: Prophylactic Antibiotic Prescribing Practice of US Hematologists.
A) Shown is the distribution of US hematologists (n=56) prescribing penicillin
versus amoxicillin as the first-line drug for S. pneumoniae prophylaxis.
Data are shown as the mean ± standard deviation. The Fisher’s exact test
was applied to generate p values. Abbreviations: Amox: Amoxicillin; PCN:
Penicillin.
B) The data for the US hematologists were analyzed by practice site setting
to determine if there was an association between practice setting and the rate
of prescribing amoxicillin versus penicillin for antibiotic prophylaxis in children
with SCD.
Discussion
The increased risk for invasive pneumococcal disease in infants with SCD and the benefits of antibiotic prophylaxis has been well established [15,17]. The goal of our study was to evaluate the use of antibiotic prophylaxis in children with SCD by hematologists in different practice settings. Despite current recommendations for S.pneumoniae prophylaxis we observed that a significant number of hematologists do not initiate therapy according to guidelines. We recently documented invasive S.pneumoniae disease in a child with SCD previously immunized with the 23-valent S.pneumoniae vaccine [18]. Although there are published evidence-based guidelines for the surveillance, initiation, and discontinuation of antibiotic prophylaxis, we observed considerable variation in clinical practices among pediatric hematologists.
In the PROPS-I study, the prevention of invasive S.pneumonia infection was demonstrated with the daily administration of penicillin to children with SCD starting early in infancy [15]. Another major impact of the PROPS-I study was the recommendation for newborn screening to diagnose SCD at birth and the initiation of penicillin prophylaxis by 4 months of age. Thus based on this guideline universal screening for hemoglobinopathies has been established for all US states and territories and many other countries worldwide. Despite the documented efficacy of penicillin, a significant number of hematologists reported using amoxicillin as the first-line drug in the absence of clinical trials in sickle cell patients to support this prescribing practice. Studies to determine S.pneumoniae patterns of drug resistance [19] demonstrated that amoxicillin, amoxicillinclavulanate and clindamycin are most effective for treating respiratory tract infections caused by this pathogen with >90% susceptibility. However, introduction of the 7- and 13-valent S.pneumoniae conjugate vaccines has altered microbial resistance patterns. Since 1998, there has been an increase in S.pneumoniae resistance with 15% and 18.9% non-susceptibility to penicillin and amoxicillinclavulanate respectively [20]. These data support penicillin as the best first-line antimicrobial for S.pneumoniae prophylaxis in patients with SCD.
The PROP-II study provided the best evidence that penicillin prophylaxis after 5 years of age does not provide additional protection against pneumococcal infection [17]. The potential of continuing penicillin prophylaxis to promote the development of S.pneumonia resistant was raised as a consideration in children with SCD continued on treatment for longer than 5 years. In our survey 27% of pediatric hematologists continue antibiotic prophylaxis after 5 years, a rate similar to those observed in a recent study by McCavit et al. [21]. They found this practice was associated with low concern about S.pneumoniae antibiotic resistance; however, the rate of resistance to penicillin continues to rise in the US reaching 15% of isolates in 2011 [20].
Our data suggest a difference in the prescribing practices of US private practice versus academic hematologists however a larger sample size is required to confirm this finding. Furthermore, the survey was only made available to pediatric hematologists attending a national Sickle Cell Disease Symposium which does not representative a cross-section of US private or rural practice hematologists, however in contrast to adult medicine, the majority of pediatric hematologists practice in an academic setting. Another limitation of our study was the low representation of hematologists from western US most likely due to the meeting location in Miami, FL. Despite these limitations, our findings provide insights into the current practice for S.pneumoniae prophylaxis in the US. While we identified factors associated with antibiotic choice among pediatric hematologists, the long-term impacts of these practices on the morbidity of children with SCD is unknown.
More recent findings related to the effects of antibiotic on the gut microbiome should be considered when weighing the potential detrimental effects of long-term penicillin or amoxicillin prophylaxis in children with SCD. Janczyk and colleagues demonstrated a reduction in the piglet intestinal bacterial diversity after a single parenteral dose of amoxicillin [22]. With the advent of new technologies such as the 16sRNA [23] and HITchip [24] arrays, detailed phylogenetic analysis of gut microbiota is now possible. Recent studies of neutrophil function demonstrated an increase in the number of circulating aged neutrophils in patients with SCD compared to healthy controls. This overly active subset of neutrophils exhibit enhanced Mac-1 activation. In contrast, penicillin prophylaxis reduced the number of circulating aged neutrophils [25]. The impact of these findings on clinical phenotypes in SCD is not known. Additional studies to address whether long-term administration of penicillin or amoxicillin alters neutrophil function and/or the composition of the gut microbiome are indicated. Understanding these changes will impact the standard of care for patients with SCD.
Acknowledgements
We would like to thank the Foundation for Sickle Cell Disease Research for providing the staff, resources and recruiting the participants who completed the survey. Special thanks to Ms. Eboni Peoples for administrative support and data collection.
References
- Platt OS, Brambilla DJ, Rosse WF, Milner PF, Castro O, Steinberg MH, et al. Mortality in sickle cell disease: Life expectancy and risk factors for early death. New Eng J Med. 1994; 330: 1639-1644.
- Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatr. 1989; 84: 500-508.
- Ballas SK, Lewis CN, Noone AM, Krasnow SH, Kamarulzaman E, Burka ER. Clinical, hematological, and biochemical features of Hb SC disease. Am J Hematol. 1982; 13: 37-51.
- River GL, Robbins AB, Schwartz SO. S-C hemoglobin: A clinical study. Blood. 1961; 18: 385-416.
- Platt OS, Thorington BD, Brambilla DJ, Milner PF, Rosse WF, Vichinsky E, et al. Pain in sickle cell disease. Rates and risk factors. N Engl J Med. 1991; 325: 11-16.
- Reed JD, Redding-Lallinger R, Orringer EP. Nutrition and sickle cell disease. Am J Hematol. 1987; 24: 441-455.
- Bandeira IC, Rocha LB, Barbosa MC, Elias DB, Querioz JA, Freitas MV, et al. Chronic Inflammatory State in sickle cell anemia patients is associated with HBB*S haplotype. Cytokine. 2014; 65: 217-221.
- National Heart, Lung, and Blood Institute. Management and Therapy of Sickle Cell Disease.
- Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010; 115: 3447- 3452.
- Overturf GD, Powars D, Baraff LJ. Bacterial meningitis and septicemia in sickle cell disease. Am J Dis Child. 1977; 131: 784-787.
- Williams TN, Uyoga S, Macharia A, Ndila C, McAuley CF, Opi DH, et al. Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. Lancet. 2009; 374: 1364-1370.
- Rogers ZR, Wang WC, Luo Z, Iyer RV, Shalaby-Rana E, Dertinger SD, et al. BABY HUG. Biomarkers of splenic function in infants with sickle cell anemia: baseline data from the BABY HUG Trial. Blood. 2011; 117: 2614-2617.
- Ram S, Lewis LA, Rice PA. Infections of People with Complement Deficiencies and Patients Who Have Undergone Splenectomy. Clinical Microbiology Reviews. 2010; 23: 740-780.
- Bjornson AB, Lobel JS. Direct evidence that decreased serum opsonization of Streptococcus Pneumoniae via the alternative complement pathway in sickle cell disease is related to antibody deficiency. J Clin Invest. 1987; 79: 388-398.
- Gaston MH, Verter JI, Woods G, Pegelow C, Kelleher J, Presbury G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. N Engl J Med. 1986; 314: 1593-1599.
- Bonds DR. Three decades of innovation in the management of sickle cell disease: the road to understanding the sickle cell disease clinical phenotype. Blood Rev. 2005; 19: 99-110.
- Falletta JM, Woods GM, Verter JI, Buchanan GR, Pegelow CH, Iyer RV, et al. Discontinuing penicillin prophylaxis in children with sickle cell anemia. J Pediatr. 1995; 127: 685-686.
- Clay EL, Burrell T, Belhorn T, Redding-Lallinger R. Immunogenicity of pneumococcal vaccination in a patient with sickle hemoglobinopathy: A case report. Clinical Case Reports. 2015; 3: 618-621.
- Jacobs MR. Streptococcus Pneumoniae: epidemiology and patterns of resistance. Am J Med. 2004; 117: 3S-15S.
- Jones RN, Sader HS, Mendes RE, Flamm RK. Update on antimicrobial susceptibility trends among Streptococcus Pneumoniae in the United States: report of ceftaroline activity from the SENTRY antimicrobial Surveillance Program (1998-2011). Diagn Microbiol Infect Dis. 2013; 75: 107-109.
- McCavit TL, Gilbert M, Buchanan GR. Prophylactic penicillin after 5 years of age in patients with sickle cell disease: a survey of sickle cell disease experts. Pediatr Blood Cancer. 2013; 60: 935-939.
- Janczyk P, Pieper R, Souffrant WB, Bimczok D, Rothkötter HJ, Smidt H. Parenteral long-acting amoxicillin reduces intestinal bacterial community diversity in piglets even 5 weeks after the administration. ISME J. 2007; 2: 180-183.
- Wagner Mackenzie B, Waite DW, Taylor MW. Evaluating variation in human gut microbiota profiles due to the DNA extraction method and inter-subject differences. Front Microbiol. 2015; 6: 130.
- Zhang D, Chen G, Manwani D, Mortha A, Xu C, Faith J, et al. Targeting Neutrophil Aging and the Microbiota for the Treatment of Sickle Cell Disease. Blood. 2015; 126: 279.
- Rajilic-Stojanovic M, Heilig HG, Molenaar D, Kajander K, Surakka A, Smidt H, et al. Development and application of the human intestinal tract chip, a phylogenetic microarray: Analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ Microbiol. 2009; 11: 1736-1751.